Document Type
Emerging StandardPublished date
06/29/2024Input Close Date
To be determinedScientific Experts
1. Introduction
To jump-start the standard development process and have earlier stakeholder engagement, USP is piloting a new approach for developing and sharing information with our stakeholders. Through a collaboration between USP’s Small Molecules Department and Global Analytical Development Laboratory, methods are being developed and validated for drug substances and drug products for which no monographs are currently available. The Emerging Standards are intended to improve USP’s official standards elaboration process by increasing transparency and allowing for broader stakeholder participation by publishing on the USP website prior to formal notice and comment through publication in the Pharmacopeial Forum.
Flunisolide Nasal Spray has been evaluated and shown to be a suitable candidate for development under this new program. The methods, in this document, are being published to help analyze Flunisolide Nasal Spray. Additional method development and validation information is provided to justify the use of method parameters.
Certain commercial software, instruments, or material may be identified in this document to specify adequately the experimental procedure. Such identification does not imply approval, endorsement, or certification by USP of a particular brand or product, nor does it imply that the software, instrument, or material is necessarily the best available for the purpose or that any other brand or product was judged to be unsatisfactory or inadequate.
This document is not a USP compendial standard and is intended to serve as a resource for informational purposes only. It does not reflect USP or USP’s Expert Body opinions of future revisions to the official text of the USP-NF. Parties relying on the information in this document bear independent responsibility for awareness of, and compliance with, any applicable federal, state, or local laws and requirements.
2. Background
Flunisolide is an inhaled corticosteroid with anti-inflammatory actions and used in the treatment of asthma. It is often prescribed as treatment for allergic rhinitis and its principal mechanism of action involves activation of glucocorticoid receptors. Flunisolide is a glucocorticoid receptor agonist. It is administered either as an oral metered-dose inhaler for the treatment of asthma or as a nasal spray for treating allergic rhinitis. Corticosteroids are naturally occurring hormones that prevent or suppress inflammation and immune responses. When given as an intranasal spray, flunisolide reduces watery nasal discharge (rhinorrhea), nasal congestion, postnasal drip, sneezing, and itching at the back of the throat that are common allergic symptoms1.
This document summarizes method validation, as well as robustness and forced degradation study results. It also describes the final procedures that may be suitable for the identification, and the strength and purity determination of flunisolide in the presence of various impurities and excipients in Flunisolide Nasal Spray. Summary of validation data and representative chromatographic results are included.
3. Materials
3.1 Flunisolide and Impurities Standards
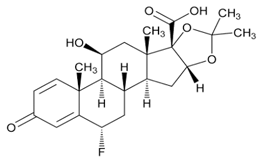
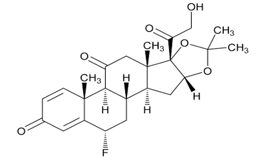
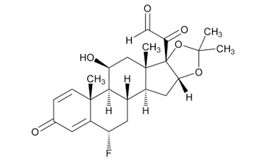
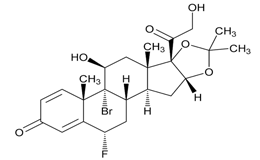
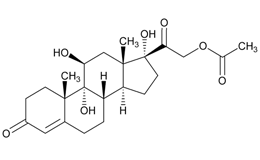
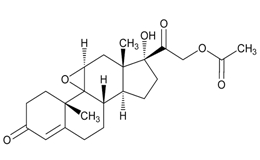
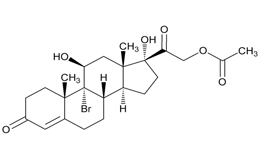
USP Reference Standards for flunisolide, desonide, flurandrenolide were used. Flunisolide 17 beta acid (related compound A), flunisolide 11-keto (related compound B), flunisolide 21-aldehyde (related compound C), 9 alfa bromo flunisolide (related compound D), dihydroxycorticosterone acetate, epoxyhydrocortisone acetate and bromohydrocortisone acetate were procured from another supplier. Chemical structures of flunisolide and related impurities are shown in Figures 1 and 2.
Figure 2: Flunisolide Impurities
3.2 Samples
Flunisolide Nasal Spray from one commercial source was used to evaluate methods described in this document.
3.3 Reagents
Potassium dihydrogen phosphate (ACS grade) was obtained from Merck and acetonitrile (HPLC grade) was from Qualigens. Water was obtained from the Sartorius water purification system.
4. Forced Degradation Study
Forced degradation study was performed by exposing flunisolide API to acid, base, oxidation, heat, humidity, and light conditions. The stressed samples and a control sample (unstressed) were analyzed and compared. The chromatograms were processed at 245 nm to detect the degradation impurities of flunisolide. The PDA data from 200-400 nm showed homogeneity of UV spectrum for the flunisolide peak indicating the peak is free from coelution. The obtained results for each of the forced degradation condition are presented in Table 1.
| Degradation | Conditions | Impurities (%) | Major degradant in % (Name of Impurity/RRT) |
|---|---|---|---|
| Control (unstressed) | Unstressed | NA | NA |
| Acidic | 0.1 N HCl; 3 days | 1.49 % | 0.58 %- (Major unknown at 0.40) 0.13% - (Flunisolide 21 aldehyde at 0.85) 0.43 %- (Desonide at 0.91) 0.25% - (Flunisolide 11 keto at 1.08) |
| Basic | Base (0.001 NaOH for 6 hours) | 8.77 % | 0.26%- (Major unknown at 0.23) 2.62 %- (Flunisolide 17 beta acid at 0.37) 3.84%- (Major unknown at 0.45) 0.75% - (Flunisolide 21 aldehyde at 0.85) 0.41 %- (Desonide at 0.91) 0.23% - (Flunisolide 11 keto at 1.08) |
| Oxidative | 3% H2O2; 3 days | 1.16 % | 0.19 %- (Flunisolide 17 beta acid at 0.36) 0.44 %- (Desonide at 0.91) 0.33% - (Flunisolide 11 keto at 1.08) |
| AIBNa | 0.5 mg/mL AIBN at 40˚C for 3 days | 1.38 % | 0.44 %- (Desonide at 0.91) 0.72 % - (Flunisolide 11 keto at 1.08) |
| Heat | 105 ˚C; 3 days | 1.07 % | 0.45 %- (Desonide at 0.91) 0.27 % - (Flunisolide 11 keto at 1.08) |
| Heat/Humidity | 85 ˚C & 85% RH; 3 days | 0.91 % | 0.44 %- (Desonide at 0.91) 0.25 % - (Flunisolide 11 keto at 1.08) |
| Light | UV exposure ≥600-watt hours/square meter (3 x ICH), Visible light exposure: ≥1.2 million lux hours (1× ICH) to occur consecutively for 3 days | 2.66 % | 0.35 %- (Flunisolide 17 beta acid at 0.36) 0.67% - (Flunisolide 21 aldehyde at 0.85) 0.40 %- (Desonide at 0.91) 0.53% - (Flunisolide 11 keto at 1.08) |
| aAIBN = Azobisisobutyronitrile | |||
In summary, desonide and flunisolide 11 keto are formed in all the above degradation conditions. Flunisolide 17 beta acid is another known degradant present in stressed samples under base, hydrogen peroxide and light exposures. One major degradation impurity was formed at 0.45 RRT (about 3.84%) under base degradation conditions. All known and unknown impurity peaks are separated from each other and no coelution was observed with the main peak.
5. Identification
Identification of flunisolide in Flunisolide Nasal Spray was evaluated using the HPLC conditions from the Assay section with PDA spectral match and chromatographic retention time match. See the Assay section for method conditions and solution preparations.
5.1 PDA Spectral Match
The validation parameters and results are summarized in Table 2, and representative UV spectra of flunisolide from the Standard solution and Sample solution are shown in Figures 3-4.
| Parameter | Solutions | Results |
|---|---|---|
| Spectral Agreement | Collect PDA data from 200 to 400 nm for the Standard solution and Sample solution. | The UV spectrum of the flunisolide peak from the Sample solution matched the spectrum of flunisolide in the Standard solution and exhibited maxima and minima only at the same wavelengths as the Standard solution. |
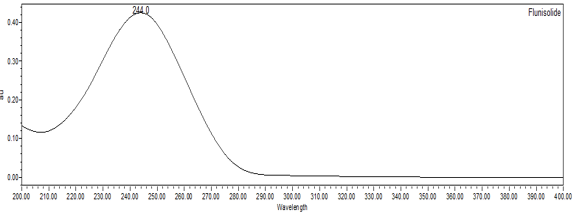
5.2 Retention Time Match
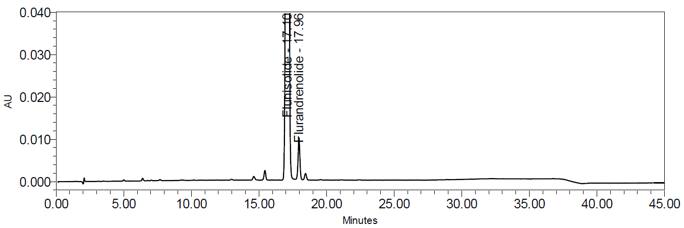
The validation parameters and results are summarized in Table 3, and representative chromatograms of the Standard solution and Sample solution are shown in Figures 5 and 6, respectively.
| Parameter | Solutions | Results |
|---|---|---|
| Retention Time Match | Standard solution and Sample solution | The relative standard deviation (RSD) of the flunisolide peak retention time for all injections of the Standard solution and Sample solution was <1.0%. |
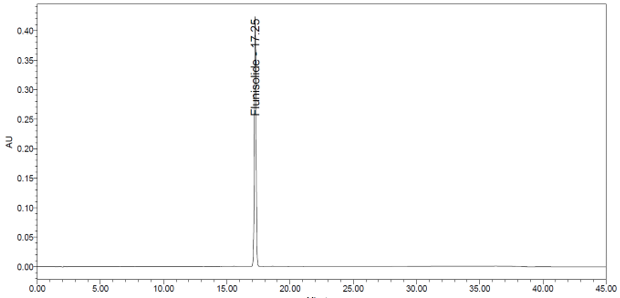
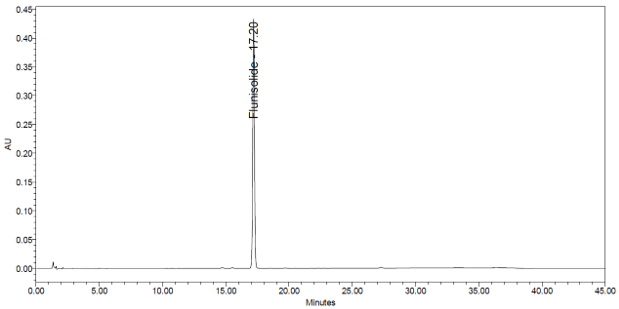
6. Assay
The validated assay procedure for Flunisolide Nasal Spray, using the criteria described in USP General Chapter <1225> Validation of Compendial Procedures2 and found to be specific, linear, accurate, precise for the samples evaluated.
6.1 Instruments and Method
The analysis of Flunisolide Nasal Spray was done using Waters Alliance 2695 and Agilent 1260 Infinity series equipped with a photo diode array (PDA) detector. Luna phenyl hexyl, 4.6-mm x 15.0-cm, 3 µm (L11) column from Phenomenex was used. The analysis was performed at 30 ˚C column temperature, with a flow rate of 1.0 mL/min and 50 µL as the injection volume. Autosampler temperature was kept at 5 ˚C. The PDA detector was set at 200-400 nm wavelength and the detection wavelength was at 245 nm with run time of 45 minutes. The separation was achieved by a gradient program as mentioned below.
| Time (Min) | Mobile Phase A (%) | Mobile Phase B (%) |
|---|---|---|
| 0 | 75 | 25 |
| 2 | 75 | 25 |
| 25 | 55 | 45 |
| 30 | 40 | 60 |
| 35 | 40 | 60 |
| 36 | 75 | 25 |
| 45 | 75 | 25 |
6.2 Solutions
Mobile Phase A (20 mM potassium dihydrogen phosphate): Weighed and transferred about 10.88 g of potassium dihydrogen phosphate into 4000 mL of water.
Mobile Phase B: Acetonitrile
Diluent: Mixed 800 mL of water and 200 mL of acetonitrile (Diluent is used as a blank).
Flurandrenolide stock solution-1 (250 µg/mL of flurandrenolide): Weighed and transferred about 1.25 mg of flurandrenolide into 5 mL volumetric flask, added 1 mL of acetonitrile, sonicated to dissolve, and diluted to volume with water.
Flurandrenolide stock solution-2 (2.5 µg/mL of flurandrenolide): Transferred 0.5 mL of the Flurandrenolide stock solution-1 into 50 mL volumetric flask and diluted to volume with Diluent.
System suitability solution (125 µg/mL of flunisolide and 1.25 µg/mL of flurandrenolide): Weighed and transferred about 1.25 mg of flunisolide into a 10 mL volumetric flask, added 1.0 mL of acetonitrile, sonicated to dissolve and added 5.0 mL of the Flurandrenolide stock solution-2 and diluted to volume with water.
Standard stock solution (250 µg/mL of flunisolide): Weighed and transferred about 12.5 mg of flunisolide standard into a 50 mL volumetric flask, added 10 mL of acetonitrile, sonicated to dissolve, and diluted to volume with water.
Standard solution (40 µg/mL of flunisolide): Transferred 4.0 mL of the Standard stock solution into 25 mL volumetric flask and diluted to volume with Diluent.
Sample solution (40 µg/mL of flunisolide): Nominally 40 µg/mL of flunisolide in Diluent prepared as follows. Prepared a composite by mixing NLT 2 containers of non-pressurized liquid drug dosage form of Nasal Spray by removing the metered-dose cap and pouring the Nasal Spray solution out of the commercialized containers into the suitable container. Transferred 4.0 mL of the composite solution (each mL is equivalent to 0.25 mg of flunisolide) into 25 mL volumetric flask and diluted to volume with Diluent.
6.3 Validation Parameters and Results
The optimized chromatographic conditions were checked for robustness and validated by evaluating specificity, linearity, precision, and accuracy as described in USP General Chapter <1225> Validation of Compendial Procedure2. The system suitability requirements and results are summarized in Table 4. The validation parameters, solutions, and results for Flunisolide Nasal Spray are summarized in Table 5. Representative chromatograms of the Standard solution and Sample solution are shown in Figures 5 and 6, respectively.
| Parameter | Solutions | Results |
|---|---|---|
| Retention time Flunisolide Flurandrenolide | System suitability solution | about 17.2 min 18.1 min |
| Relative retention time Flunisolide Flurandrenolide | about 1.00 1.05 | |
| Resolution Resolution between Flunisolide and Flurandrenolide (NLT 2.0) | Resolution was 3.6 | |
| System Precision (RSD of 5 replicate injections NMT 0.5%) Flunisolide | Standard solution | RSD was 0.05% |
| USP Tailing Flunisolide (NLT 0.8 and NMT 1.8) | Tailing was 1.01 | |
| Abbreviations: RSD: relative standard deviation, NLT: Not less than, NMT: Not more than | ||
| Parameter | Solutions | Results |
|---|---|---|
Specificity
Peak Purity Analysis | Diluent, System suitability solution, Standard solution, and Sample solutions | Diluent has no interference with the flunisolide peak. No peak (≥0.1% total area) is detected from the Standard solution and Sample solutions of drug product. The PDA data from 200-400 nm showed homogeneity of the UV spectrum for the flunisolide peak, indicating that there is no coelution. |
| Linearity | Linearity solutions from 50 % to 150 % of the sample concentration (0.02, 0.03, 0.04, 0.05, 0.06 mg/mL of flunisolide). | The correlation coefficient (r) was not less than 0.999. The bias of the linearity curve due to the intercept not being zero was within ±2.0%. |
| Accuracy | Accuracy solutions from 110-130% of the sample concentration were prepared in triplicate at each level. 10% spiked (0.044 mg/mL), n=3 20% spiked (0.048 mg/mL), n=3 30% spiked (0.052 mg/mL), n=3 | The average recovery result at each spiked level was within 100 ± 3.0% when compared to the standard solution. |
| Repeatability | Repeatability solutions: 6 Sample solutions | The % RSD was NMT 2.0 (n=6) |
| Intermediate Precision | 6 Sample solutions prepared and analyzed by a different analyst, on a different day by using different instrument and using a different serial number of the column | The % RSD was NMT 2.0 (n=6) The % RSD was NMT 3.0 for the combined data of the first and second analysts (n =12) |
| Solution Stability | Standard and Sample solutions | Standard and sample solutions were stable for 48 hours at 5°C autosampler temperature. |
| Sample Assay | Sample solutions | 101.5% for the drug product tested. |
| Retention Time Match | Standard and Sample solutions | The relative standard deviation (%RSD) of flunisolide peak retention time for all injections of the Standard solution and sample solutions was less than 1.0% |
7. Organic Impurities
The developed HPLC method was validated for the OI procedure, using the criteria described in USP General Chapter <1225> Validation of Compendial Procedure2 and found to be specific, accurate, precise, robust, linear, and free from interference for the samples evaluated.
Bromo hydrocortisone is a process impurity which is not controlled in the drug product. The same method could be used for analysis of bromo hydrocortisone, if needed (RRT about 1.3). However, due to the instability of the impurity in the solution, the standards and samples should be freshly prepared and analyzed within 1 hour of preparation.
7.1 Instruments and method
The analysis of Flunisolide Nasal Spray was performed using the same instruments and method as described in the Assay section.
7.2 Solutions
Prepare Mobile Phase A, Mobile Phase-B, Diluent and System suitability solution as per the Assay procedure.
Preparation of Individual stock solutions: Individual stock solutions consisting of 250 µg/mL flunisolide 17 beta acid, dihydroxycorticosterone acetate, flunisolide 21-aldehyde, desonide, flunisolide, flurandrenolide, flunisolide 11-keto, epoxyhydrocortisone acetate and 9 alfa bromo flunisolide were individually prepared by dissolving appropriate amounts of each standard in Diluent.
Preparation of Sensitivity solution: A solution consisting of 0.0625 µg/mL each of flunisolide 17 beta acid, dihydroxycorticosterone acetate, flunisolide 21-aldehyde, desonide, flunisolide, flurandrenolide, flunisolide 11-keto, epoxyhydrocortisone acetate and 9 alfa bromo flunisolide was prepared by combining appropriate volumes of Individual stock solutions in Diluent.
Preparation of Standard solution: A solution consisting of 0.125 µg/mL each of flunisolide
17 beta acid, dihydroxycorticosterone acetate, flunisolide, flurandrenolide, flunisolide 11-keto, epoxyhydrocortisone acetate, 9 alfa bromo flunisolide and 0.5 µg/mL for desonide and flunisolide 21 aldehyde was prepared by combining appropriate volumes of Individual stock solutions in Diluent.
Preparation of Sample solution: Nominally 125 µg/mL of flunisolide in Diluent prepared as follows. Prepared a composite by mixing NLT 2 containers of non-pressurized liquid drug dosage form of Nasal Spray by removing the metered-dose cap and pouring the Nasal Spray solution out of the commercialized containers into the suitable container. Transferred 2.5 mL of the composite solution (each mL is equivalent to 0.25 mg of flunisolide) into 5 mL volumetric flask, added 1 mL of acetonitrile and diluted to volume with water.
Preparation of Robustness solution: A solution consisting of 125 µg/mL of flunisolide and 1.25 µg/mL of each of flunisolide 17 beta acid, dihydroxycorticosterone acetate, flurandrenolide, flunisolide 11-keto, epoxyhydrocortisone acetate, 9 alfa bromo flunisolide and 0.5 µg/mL for desonide and flunisolide 21 aldehyde was prepared by combining appropriate volumes of Individual stock solutions in Diluent.
7.3 Validation Parameters and Results
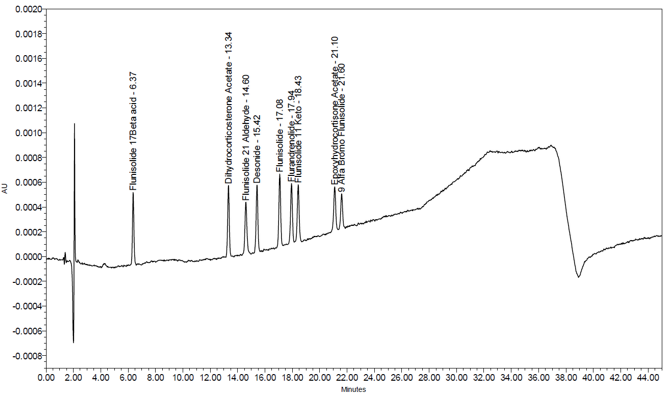
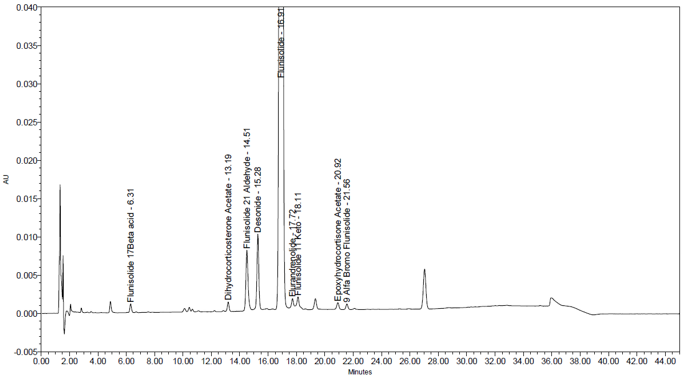
The system suitability parameters and results are summarized in Table 6. The validation parameters and results are summarized in Table 7. Representative chromatograms of Blank, System suitability solution, Sensitivity solution, Spiked sample solution, and Sample solution are presented in Figures 7-11. Linearity was established for flunisolide, and related impurities and relative response factors presented in Table 8, whereas accuracy and repeatability were established for flunisolide related impurities.
The Sensitivity solution was considered as limit of quantitation (LOQ) for each analyte at 0.0625 µg/mL with respect to the sample concentration of 125 µg/mL.
| Parameter | Solution | Results |
|---|---|---|
| Retention time (mins) Flunisolide Flurandrenolide | System suitability solution | about 17.1 18.0 See Figure 8 |
| Relative retention time Flunisolide Flurandrenolide | 1.00 1.05 | |
| Resolution Resolution between Flunisolide and Flurandrenolide (NLT 2.0) | 3.57 | |
| Retention time (mins) Flunisolide 17 beta acid Dihydroxy corticosterone acetate Flunisolide 21-aldehyde Desonide Flunisolide Flurandrenolide Flunisolide 11-keto Epoxy hydrocortisone acetate 9 alfa bromo flunisolide | Sensitivity solution (0.05%) | about 6.4 13.3 14.6 15.4 17.1 17.9 18.4 21.1 21.6 See Figure 9 |
| Relative retention time Flunisolide 17 beta acid Dihydroxy corticosterone acetate Flunisolide 21-aldehyde Desonide Flunisolide Flurandrenolide Flunisolide 11-keto Epoxy hydrocortisone acetate 9 alfa bromo flunisolide (RC D) | 0.37 0.78 0.85 0.90 1.00 1.05 1.08 1.23 1.26 | |
| System Precision (%RSD of 6 replicate injections NMT 5.0) Flunisolide 17 beta acid Dihydroxy corticosterone acetate Flunisolide 21-aldehyde Desonide Flunisolide Flurandrenolide Flunisolide 11-keto Epoxy hydrocortisone acetate 9 alfa bromo flunisolide | 3.42 0.86 3.63 1.27 1.14 1.89 2.14 2.06 3.12 | |
| USP Signal-to-Noise ratio Flunisolide 17 beta acid Dihydroxy corticosterone acetate Flunisolide 21-aldehyde Desonide Flunisolide Flurandrenolide Flunisolide 11-keto Epoxy hydrocortisone acetate 9 alfa bromo flunisolide | 84 70 60 60 53 58 57 58 56 |
| Parameter | Solutions | Results |
|---|---|---|
| Specificity | Blank (Diluent), System suitability solution, Sensitivity solution, Sample solutions and Spiked sample solutions. | No interference to peaks of interest. |
Linearity Flunisolide 17 beta acid | Linearity solutions: 0.05%, 0.10%, 0.20%, 0.50%, 0.75%, 1.00%, 1.50% and 2.00% of the sample concentration. | The correlation coefficients of the linear curves for Flunisolide and related impurities were ≥0.99. |
| Relative Response Factor (RRF) Values | RRF values of the impurities were calculated with respect to flunisolide. The values were obtained by dividing the slope of the linearity curve for the impurity by the slope of the linearity curve for the flunisolide. | See Table 8. |
Accuracy Flunisolide 17 beta acid | Accuracy solutions: Impurities spiked in sample solution at 3 levels; Recovery lower level (RLL)*: n=6 Recovery middle level (RML) (1.0%): n=3 Recovery upper level (RUL) (2.0%): n=3 *RLL Spiked sample solution contains 0.1% of all impurities and 0.4% of desonide and flunisolide 21-aldehyde. | The average recovery for each specified impurity at each level were observed within: RLL (0.1% of all impurities and 0.4% of desonide and flunisolide 21-aldehyde): 100 ± 20.0%. RML (1.0%): 100 ±10.0%. RUL (2.0%): 100 ± 5.0%. |
Repeatability Flunisolide 17 beta acid | Repeatability solutions: 6 Spiked sample solutions at the RLL level | The %RSD of the recovery was ≤ 10.0 (n=6) |
Intermediate Precision Flunisolide 17 beta acid | Repeatability solutions: 6 Spiked sample solutions at the RLL level Prepared and evaluated by a different analyst, on a different day by using different instrument and using a different serial number of the column. | The average recovery at RLL was within 100 ± 20.0%. %RSD of the 6 results at RLL was ≤ 10.0. %RSD of the 12 results (for the 2 analysts) at RLL was ≤15.0. |
| Sample Analysis | Two Sample solutions from sample material | About 0.41% w/w of Desonide, 0.46% w/w of Flunisolide 21 aldehyde and about 0.55 % w/w of one unspecified impurity was observed, and no other impurity above 0.2% w/w was observed in sample. See Figure 11 and Table 9. |
Solution Stability Flunisolide 17 beta acid | Sensitivity solution at 0.1% and Spiked sample solutions (at 0.1% level), freshly prepared and analyzed periodically over 48 hours at 5°C of auto sampler temperature. | Observed changes in the peak area from both solutions for each specified impurity (except flunisolide 17 beta acid and flunisolide 21 aldehyde) and flunisolide were within ± 10% of the initial time point values up to 48 hours. Flunisolide 17 beta acid is found to be stable up to 20 hours. and flunisolide 21 aldehyde is found to be stable up to 16 hours at 5°C. |
| Robustness | Robustness solution Changes evaluated: Flow rate 1.0 ± 0.1 mL/min, Column temperature 30°C ± 3°C, initial hold time (±0.5 min), minor component at post gradient by ± 2% (absolute) | System suitability criteria for resolution, retention time and relative retention time met across all evaluated conditions. |
| Name | RRF | |
| 1 | Flunisolide 17 beta acid | 0.82 |
| 2 | Dihydroxy corticosterone acetate | 0.91 |
| 3 | Flunisolide 21-aldehyde | 0.81 |
| 4 | Desonide | 0.91 |
| 5 | Flurandrenolide | 0.85 |
| 6 | Flunisolide 11-keto | 0.87 |
| 7 | Epoxy hydrocortisone acetate | 0.69 |
| 8 | 9 alfa bromo flunisolide | 0.52 |
| 9 | Bromohydrocortisone acetate | 0.63 |
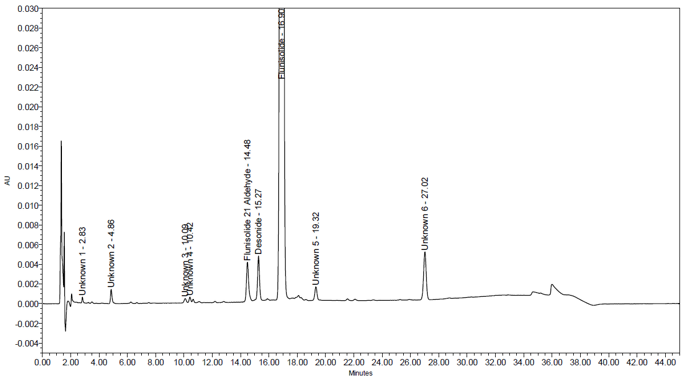
| Drug Product Sample Analysis | |||
| Name of Source | Type of Impurity | Name of Impurity | %Impurity |
| Flunisolide Nasal Spray | Unspecified | Unknown -1 | 0.03 |
| Unspecified | Unknown -2 | 0.09 | |
| Unspecified | Unknown -3 | 0.04 | |
| Unspecified | Unknown -4 | 0.03 | |
| Unspecified | Unknown -5 | 0.13 | |
| Unspecified | Unknown -6 | 0.55 | |
| Specified | Flunisolide 21 aldehyde | 0.46 | |
| Specified | Desonide | 0.41 | |
| Total Impurity | 1.74 | ||
Comment On [Methods for the Analysis of Flunisolide Nasal Spray]
Submitted on version:
Comment On [Methods for the Analysis of Flunisolide Nasal Spray]
Submitted on version:
Comment On [Methods for the Analysis of Flunisolide Nasal Spray]
This is a test comment. No file is attached.
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dol...Read more
This is a test comment. No file is attached.
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur. Excepteur sint occaecat cupidatat non proident, sunt in culpa qui officia deserunt mollit anim id est laborum.
Read lessSubmitted on version:
Comment On [Methods for the Analysis of Flunisolide Nasal Spray]
Submitted on version:
Comment On [Methods for the Analysis of Flunisolide Nasal Spray]
Submitted on version: