Document Type
Emerging StandardPublished date
11/01/2023Input Close Date
To be determinedScientific Experts
Dom Vicchio (Sr. Director, USP)
Introduction
To jump-start the consideration of the potential standards development process and have earlier stakeholder engagement, USP is piloting a new approach for developing and sharing information with our stakeholders. Through a collaboration between USP’s Small Molecules Department and the Global Analytical Development Laboratory, methods will be developed and validated for drug substances and drug products for which no monographs are currently available. These Emerging Standards are intended to improve USP’s official standards elaboration process by increasing transparency and allowing for broader stakeholder participation by publishing on the USP website prior to formal notice and comment through publication in the Pharmacopeial Forum.
Doxazosin Extended‐Release Tablets have been evaluated and shown to be a suitable candidate for development under this new program. The methods in this document are being published to help analyze Doxazosin Extended‐Release Tablets as a part of USP’s mission to improve global health through public standards that help ensure the safety, quality, and benefit of medicines and foods.
Certain commercial equipment, instruments, or materials may be identified in this document to specify adequately the experimental procedure. Such identification does not imply approval, endorsement, or certification by USP of a particular brand or product, nor does it imply that the equipment, instrument, or material is necessarily the best available for the purpose or that any other brand or product was judged to be unsatisfactory or inadequate.
This document is not a USP compendial standard and is intended to serve as a resource for information purposes only. It does not reflect USP or USP’s Expert Body opinions of future revisions to the official text of the USP‐NF. Parties relying on the information in this document bear independent responsibility for awareness of, and compliance with, any applicable federal, state, or local laws and requirements.
Background
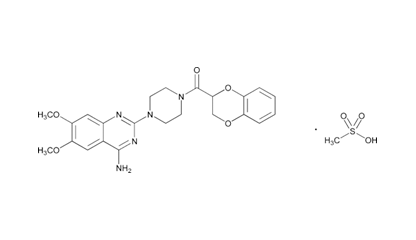
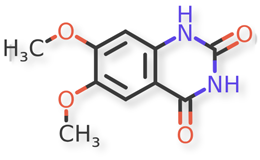
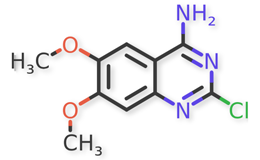
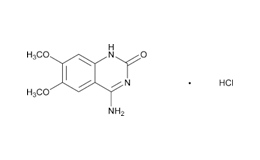
Doxazosin (Piperazine, 1-(4-amino-6,7-dimethoxy-2-quinazolinyl)-4-[(2,3-dihydro-1,4-benzodioxin-2-yl) carbonyl]-, monomethanesulfonate) is a class of medications called alpha-blockers. Doxazosin is the active ingredient in Doxazosin Extended-Release Tablets. Doxazosin is used in men to treat the symptoms of an enlarged prostate (Benign Prostatic Hyperplasia or BPH), which include difficulty urinating (hesitation, dribbling, weak stream, and incomplete bladder emptying), painful urination, and urinary frequency and urgency. It is also used alone or in combination with other medications to treat high blood pressure. It relieves the symptoms of BPH by relaxing the muscles of the bladder and prostate. It lowers blood pressure by relaxing the blood vessels so that blood can flow more easily through the body1.
The USP-NF includes a monograph for Doxazosin, which covers both the drug substance and drug product (Tablets). As a result, the decision was made to assess the appropriateness of the monograph procedures. After conducting evaluations, specific procedures are established for Doxazosin Extended-Release Tablets.
This document presents methods and provides accompanying data from chromatographic systems for achieving peak retention time match and photodiode array (PDA) spectral match, enabling the identification of doxazosin in the presence of different impurities and excipients. Additionally, the document describes High-Performance Liquid Chromatography (HPLC) methods along with validation data that are suitable for accurately determining the strength and purity of doxazosin.
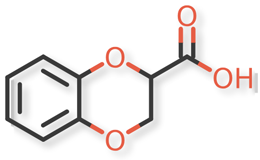
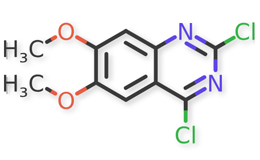
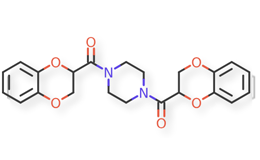
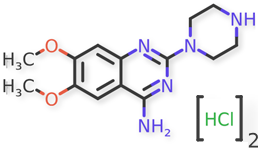
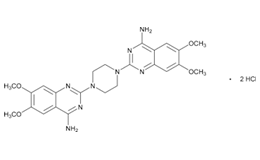
Doxazosin Mesylate and related impurities are shown in Figures 1 and 2.
EXPERIMENTAL
Materials:
Doxazosin Mesylate and related impurities (Doxazosin RC-A, Doxazosin RC-B, Doxazosin RC-C, Doxazosin RC-D, Doxazosin RC-E, Doxazosin RC-F, Doxazosin RC-G, Terazosin RC-A and Terazosin RC-C ) are available in USP catalogue, the materials are sourced from USP.
The FDA-approved source of Doxazosin Extended-Release Tablets, , was obtained and utilized for the purpose of evaluating identification through ultraviolet (UV) spectral match and retention time match, as well as conducting assay and impurity analyses using the methods described in this document.
IDENTIFICATION
The identification of doxazosin within Doxazosin Extended-Release Tablets was assessed using HPLC-UV with a photodiode array (PDA) detector, as well as by comparing the chromatographic HPLC retention time.
A. HPLC-UV with PDA Detector:
The HPLC assay procedure, incorporating a PDA detector, served as the chromatographic identification method. For specific details on the method conditions and solution preparations, please refer to the Assay section. The validation parameters and results are presented in Table 1, while Figures 3 and 4 display the UV spectra of the doxazosin standard and sample for comparison.
| Parameter | Samples and Procedure | Results |
|---|---|---|
| Spectral Agreement | Collect PDA data from 190-400 nm for the Standard solution and Sample solution | The UV spectrum of the doxazosin peak from the Sample solution matched the spectrum of doxazosin in the Standard solution and exhibited maxima and minima only at the same wavelengths as the Standard solution. |
| Abbreviation- PDA: Photodiode array; UV: ultraviolet | ||
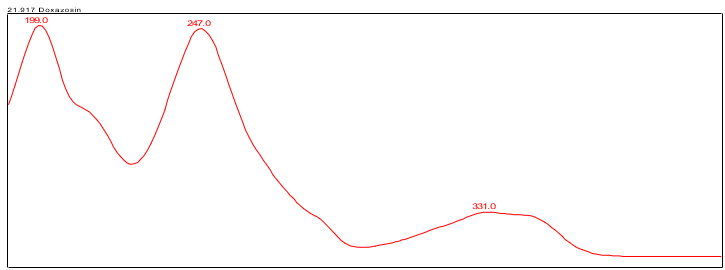
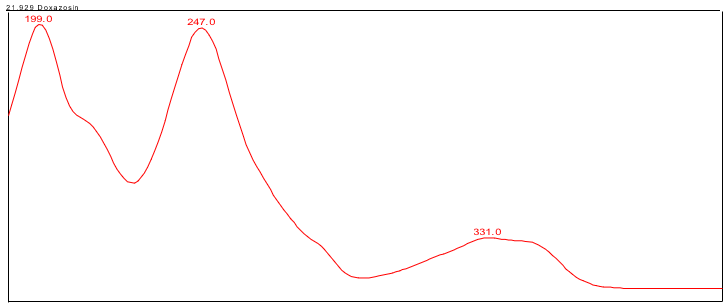
B. Retention Time Match:
To identify doxazosin, the chromatographic retention time is utilized as an alternative method. The HPLC assay procedure was employed for this identification test. For specific information regarding the method conditions and solution preparations, please refer to the Assay section.
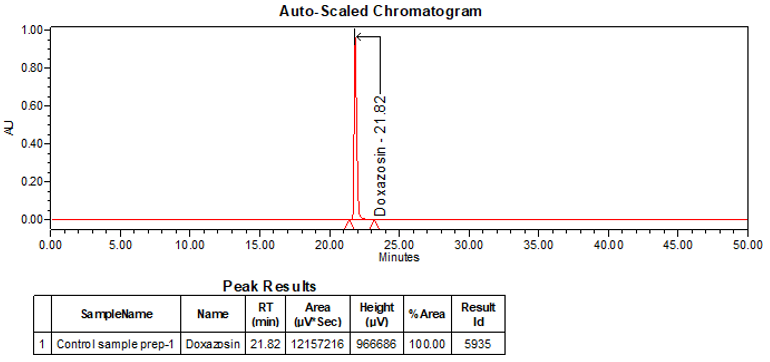
The validation parameter and corresponding results are summarized in Table 2. Additionally, Figures 5 and 6 depict the example chromatograms for the Standard solution and Sample solution, respectively.
| Parameter | Samples | Results |
|---|---|---|
| Retention Time Match | Standard solution and Sample solution | The relative standard deviation (%RSD) of the doxazosin peak retention time for all injections of the Standard solution and Sample solution was <1.0. |
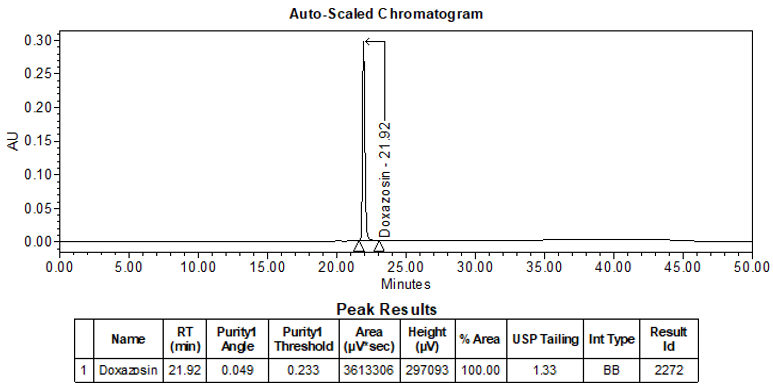
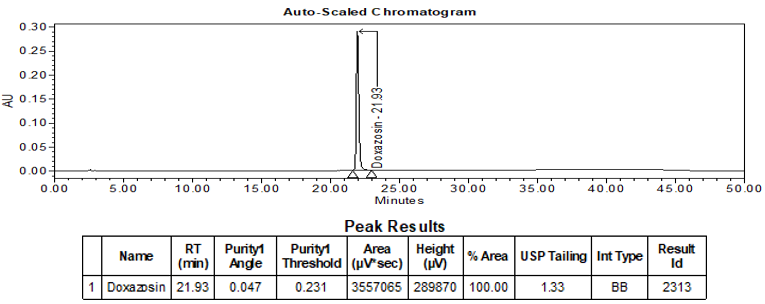
ASSAY
A gradient reversed-phase HPLC method was developed to analyze the assay and organic impurities in Doxazosin Extended-Release Tablets. This procedure was validated according to the guidelines outlined in USP General Chapter <1225>, Validation of Compendial Procedures2. It was determined to be specific, linear, accurate, precise, robust, and without any interference for the evaluated samples.
Chemicals:
Ortho phosphoric acid 88% (ACS grade) was procured from Merck. Acetonitrile (LC-MS grade) was obtained from J.T. Baker and Fisher Scientific. Ultrapure water used for HPLC analysis was obtained from a Sartorius water purification system.
Instruments and method:
The analysis of Doxazosin Extended-Release tablets was performed using Waters Alliance e2695 and Agilent infinity 1260 series with PDA detector, and the results were processed by Empower (Waters software). The LiChrospher 60 RP-Select B, 4.0-mm x 25.0-cm, 5 µm column from Merck was used for analysis. The analysis was performed at 35°C, with a flow rate of 0.8 mL/min and 10 µL as the injection volume. Autosampler temperature was kept at 10°C. The PDA detector was set at 190-400 nm wavelength and the detection of chromatogram was at 210 nm. All the solutions are protected from light and prepared in low actinic glassware. Separation was achieved by a gradient program as listed in Table 3.
| Time (min) | Solution A% | Solution B% | Solution C% |
|---|---|---|---|
| 0.0 | 20 | 10 | 70 |
| 10.0 | 20 | 22 | 58 |
| 35.0 | 20 | 50 | 30 |
| 40.0 | 20 | 50 | 30 |
| 43.0 | 20 | 10 | 70 |
| 50.0 | 20 | 10 | 70 |
Solutions:
Solution-A: 50 mg/mL (w/v) of phosphoric acid in water.
Solution-B: Acetonitrile.
Solution-C: Water.
Solution-D: Premix 100 mL of Solution B and 2 g of phosphoric acid.
Diluent: Premix Solution C and Solution D in the ratio of (9:1, v/v), sonicate to mix.
Preparation of Standard solution:
Standard solution: 0.06 mg/mL of doxazosin from USP Doxazosin Mesylate RS in Diluent.
Preparation of Sample solution:
Nominally 0.06 mg/mL of doxazosin from Doxazosin Extended-Release (ER) Tablets was prepared as follows. Crush three Doxazosin ER Tablets (equivalent to 12 mg of doxazosin), and transfer the entire amount in to 200 mL volumetric flask, to this add 100 mL of solution C and 20 mL of solution D to get dispersed. Keep the sample in Sonicator for 10 min, shake it periodically, and add about 30 mL of solution C. Keep the sample for extraction by using mechanical shaker at 300 rpm for 180 min. Dilute with solution C to volume and shake well to mix. Transfer the solution into centrifuge tube and centrifugate for 15 min at 4000 rpm. Take supernatant solution and filter through 0.45 µm PTFE Filter. Collect the sample solution by discarding initial 3 mL.
Analytical parameters and validation:
The optimized chromatographic conditions were checked for robustness and validated by evaluating specificity, linearity, precision, and accuracy as described in USP general chapter <1225>2. The system suitability requirements are summarized in Table 4. The validation parameters, solutions, and results for Doxazosin Extended-Release Tablets are summarized in Table 5. The example chromatograms for the assay Standard solution and Sample solution are shown in Figures 5 and 6, respectively.
| Parameter | Solutions | Results |
|---|---|---|
| Retention time | Standard solution | About 21.9 min |
| Tailing factor | Standard solution | Tailing factor was NMT 2.0 |
| System Precision (for 5 replicate injections) | Standard solution | %RSD was NMT 0.5 |
| Abbreviation: RSD, relative standard deviation; NMT, not more than | ||
| Parameter | Solutions | Results |
|---|---|---|
Specificity (Chromatographic Separation) Peak Purity Analysis (Spectral Homogeneity) | Blank (Diluent), Standard solution, and Sample solution | Any impurity peak from the Standard solution and Sample solution was separated from the doxazosin peak by a resolution ≥2.0 The PDA data from 190–400 nm showed homogeneity of UV spectrum for the doxazosin peak, indicating the lack of coelution. |
| Linearity | Linearity solutions from 50% to 150% of the nominal concentration (0.030, 0.045, 0.060, 0.075, and 0.090 mg/mL of doxazosin) | The correlation coefficient (r) was not less than 0.999 The bias of the linearity curve due to the intercept not being zero was less than ± 2.0% |
| Accuracy | Prepared Sample solutions spiked with three levels of the Standard solution, at 10%, 20%, and 30% of the nominal sample concentration in triplicate. 110% (0.066mg/mL), n=3 120% (0.072mg/mL), n=3 130% (0.078mg/mL), n=3 | The average recovery at each level was within 100 ± 3.0% |
| Repeatability | Repeatability solutions: 6 Sample solutions | The %RSD of the assay results was NMT 2.0 (n=6) |
| Intermediate Precision | Intermediate precision was done by a different analyst, on a different day by using a different make of instrument and different serial number of the column. | The %RSD of the assay results was NMT 2.0 (n=6) The %RSD was NMT 3.0 for the combined data of the first and second analysts (n =12) |
| Solution Stability | Standard solution and Sample solution | Standard solution and Sample solution were stable for 40 h at a sample cooler temperature of 10°C. |
| Sample Assay Test | Sample solution | 99.6% for the source of the drug product tested. |
ORGANIC IMPURITIES
The HPLC method used to analyze organic impurities in Doxazosin Extended-Release Tablets is the same as the procedure described in the Assay section. This method can effectively measure degradation products in the Doxazosin Extended-Release tablets. By following the validation criteria outlined in <1225>2, the procedure was found to be specific, accurate, precise, robust, linear, and free from interference when applied to the tested samples. The validation study verified that this method is suitable for evaluating organic impurities in the Doxazosin drug product.
Solutions:
Prepare Solution A, Solution B, Solution C, Solution D, Diluent, and follow chromatographic conditions, as per the Assay procedure.
Preparation of Impurity stock solutions:
Individual Impurity stock solutions consisting of 0.10 mg/mL each of doxazosin related compound A, doxazosin related compound B, doxazosin related compound C, doxazosin related compound D, doxazosin related compound E, doxazosin related compound F, doxazosin related compound G, terazosin related compound A, and terazosin related compound C was prepared by dissolving the appropriate amounts of each impurity separately in diluent with the aid of sonication.
Preparation of Standard stock solution 1:
Standard stock solution 1 (0.1 mg/mL of doxazosin) was prepared by dissolving an appropriate amount of Doxazosin mesylate standard in diluent.
Preparation of Standard stock solution 2:
Standard stock solution 2, containing 0.01 mg/mL of doxazosin, was prepared from the Standard stock solution 1 by further diluting with the diluent.
Preparation of System suitability solution:
A solution consisting of 0.002 mg/mL each of terazosin related compound A, doxazosin related compound G, doxazosin related compound A and doxazosin related compound B was prepared by combining appropriate volumes of each of the respective individual Impurity stock solutions in diluent.
Preparation of Peak identification solution:
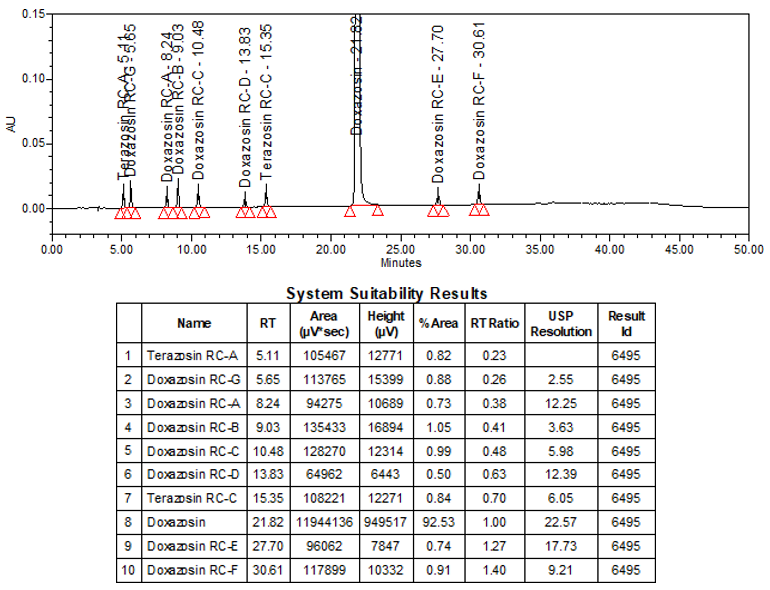
A solution consisting of 0.2 mg/mL of doxazosin from USP Doxazosin Mesylate RS and 0.002 mg/mL (or) 1% each of doxazosin related compound A, doxazosin related compound B, doxazosin related compound C, doxazosin related compound D, doxazosin related compound E, doxazosin related compound F, doxazosin related compound G, terazosin related compound A, and terazosin related compound C was prepared by combining appropriate amount of USP Doxazosin Mesylate RS with appropriate volume of each of the respective individual Impurity stock solutions in diluent.
Preparation of Impurity stock solution 1:
A solution containing 0.01 mg/mL Doxazosin related compound A, Doxazosin related compound B, Doxazosin related compound C, Doxazosin related compound D, Doxazosin related compound E, Doxazosin related compound F, Doxazosin related compound G, and Terazosin related compound A was prepared by combining appropriate volumes of each of the respective individual Impurity stock solutions in diluent.
Preparation of Sensitivity solution:
A solution consisting of 0.2 µg/mL (0.1% of the sample concentration) each of doxazosin related compound A, doxazosin related compound B, doxazosin related compound C, doxazosin related compound D, doxazosin related compound E, doxazosin related compound F, doxazosin related compound G, terazosin related compound A, and doxazosin was prepared by combining appropriate volume of Impurity stock solution 1 and Standard stock solution 2 in diluent.
Preparation of Standard solution:
A solution consisting of 0.5 µg/mL of doxazosin (0.25% of the sample concentration) was prepared from Standard stock solution 2 in diluent.
Preparation of Sample solution:
Nominally 0.2 mg/mL of doxazosin from Doxazosin Extended-Release Tablets prepared as follows.
Crush one Doxazosin Extended-Release Tablet (equivalent to 4 mg of doxazosin) and transfer the entire amount in to a 50 mL volumetric flask. Add accurately 10 mL of Solution C, and 2 mL of Solution D, to get dispersed. Keep the sample in Sonicator for 10 min, shake it periodically. Add accurately 8 mL of Solution C. Keep the sample for extraction by using mechanical shaker at 300 rpm for 180 min. Transfer the solution into a centrifuge tube and centrifugate for 15 min at 4000 rpm. Take supernatant solution and filter through 0.45 µm PTFE Filter. Collect the sample solution by discarding initial 3 mL.
Analytical parameters and validation:
The robustness of the optimized chromatographic conditions obtained from the assay was examined and validated through the assessment of specificity, linearity, accuracy, repeatability, intermediate precision, and sample analysis, following the guidelines outlined in USP General Chapter <1225>2. Linearity was determined for both doxazosin and its associated impurities, while accuracy and repeatability were established for doxazosin related impurities. Terazosin RC-C was not included in validation studies.
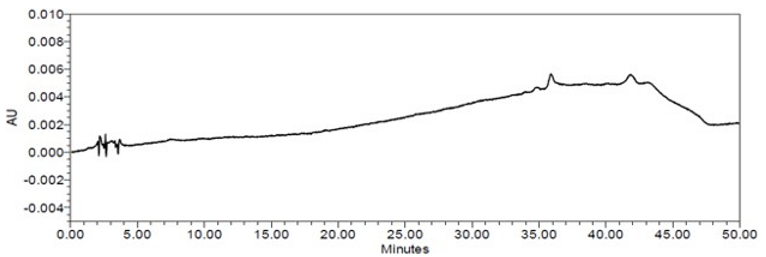
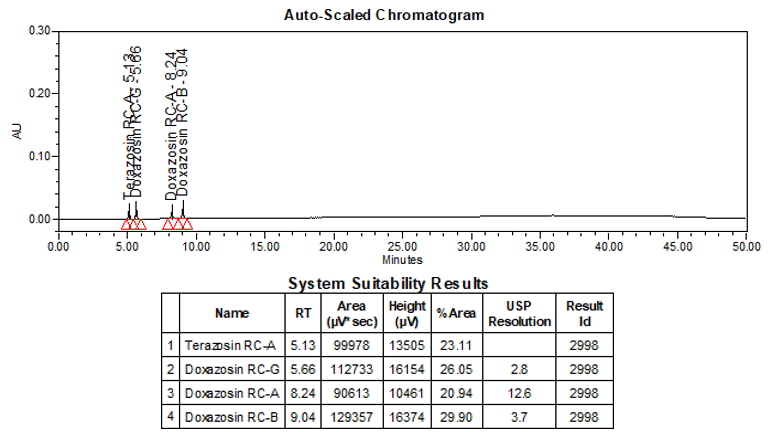
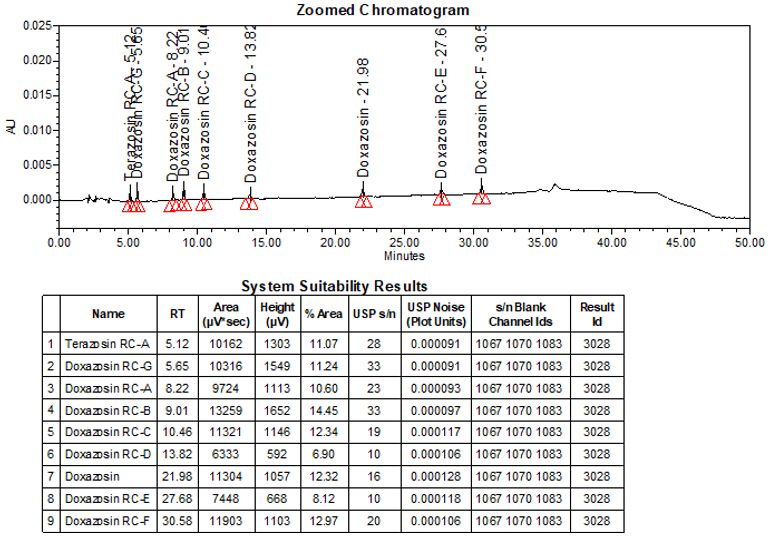
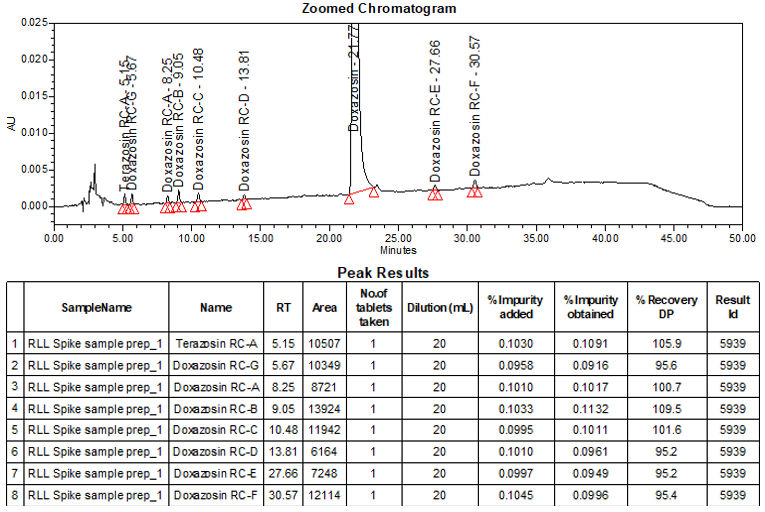
The limit of quantitation (LOQ) was established at 0.10% of sample nominal concentration. The system suitability requirements are summarized in Table 6. The validation parameters and testing results for Doxazosin Extended-Release Tablets are summarized in Table 7. The example chromatograms of Blank (Diluent), System suitability solution, Peak identification solution, Sensitivity solution (0.10%), Sample solution spiked with known impurities at sensitivity level (0.10%), and Sample solution are presented in Figures 7–12, respectively.
| Parameter | Solution | Results |
|---|---|---|
Resolution Between Terazosin RC-A and Doxazosin RC-G Between Doxazosin RC-A and Doxazosin RC-B
| System suitability solution | 2.8 3.7 (See Figure 8) |
Relative Retention Time(s) Terazosin RC-A Doxazosin RC-G Doxazosin RC-A Doxazosin RC-B Doxazosin RC-C Doxazosin RC-D Terazosin RC-C Doxazosin Doxazosin RC-E Doxazosin RC-F
| Peak identification solution | 0.23 0.26 0.38 0.41 0.48 0.63 0.70 1.00 1.27 1.40 (See Figure 9) |
System Precision (%RSD of 6 replicate injections) Terazosin RC-A Doxazosin RC-G Doxazosin RC-A Doxazosin RC-B Doxazosin RC-C Doxazosin RC-D Doxazosin Doxazosin RC-E Doxazosin RC-F | Sensitivity solution | 3.8 4.5 3.3 1.7 3.8 1.6 4.0 3.7 4.7 |
USP Signal-to-Noise Ratio Terazosin RC-A Doxazosin RC-G Doxazosin RC-A Doxazosin RC-B Doxazosin RC-C Doxazosin RC-D Doxazosin Doxazosin RC-E Doxazosin RC-F | Sensitivity solution | 28 33 23 33 19 10 16 10 20 |
| Parameter | Solution | Results |
|---|---|---|
| Specificity | Blank (Diluent), Peak identification solution, Sensitivity solution, Sample solution, and spiked Sample solution | No interference between peaks of interest. Any impurity peak ≥0.1% total area was separated from the main peak by a resolution of ≥2.0, and from adjacent specified impurity peaks by a resolution of ≥1.5. |
LOQ (0.10%) Terazosin RC-A Doxazosin RC-G Doxazosin RC-A Doxazosin RC-B Doxazosin RC-C Doxazosin RC-D Doxazosin Doxazosin RC-E Doxazosin RC-F | Sensitivity solution and accuracy solution spiked with impurities at the LOQ level. Experimentally determined using signal-to-noise values, and meeting accuracy, and repeatability criteria at that concentration. | All concentration values met a signal-to-noise criterion of ≥ 10 (refer to Figure 10). See below for accuracy and repeatability. |
Linearity Terazosin RC-A Doxazosin RC-G Doxazosin RC-A Doxazosin RC-B Doxazosin RC-C Doxazosin RC-D Doxazosin Doxazosin RC-E Doxazosin RC-F | Linearity solutions From LOQ (0.10%) to 1.0% of the nominal concentration of doxazosin and related impurities in diluent | The correlation coefficient of the linear curves for doxazosin and the impurities were not less than 0.99 |
| Relative Response Factor (RRF) Values | Linearity solutions From LOQ (0.10%) to 1.0% of the nominal concentration of doxazosin and related impurities in diluent | For results, refer to Table 8. |
Accuracy Terazosin RC-A Doxazosin RC-G Doxazosin RC-A Doxazosin RC-B Doxazosin RC-C Doxazosin RC-D Doxazosin RC-E Doxazosin RC-F | Accuracy solutions: impurities spiked in Sample solution at 3 levels: LOQ (0.10%): n=6 | The average recovery for each specified impurity at each level were observed to be within: 0.10% : 100 ± 20.0% 0.50% : 100 ±10.0% 1.0% : 100 ± 5.0% |
Repeatability Terazosin RC-A Doxazosin RC-G Doxazosin RC-A Doxazosin RC-B Doxazosin RC-C Doxazosin RC-D Doxazosin Doxazosin RC-E Doxazosin RC-F | Repeatability solutions: 6 spiked Sample solutions at the LOQ level | The %RSD of the recovery was ≤ 10.0% (n=6). See Figure 11 for example chromatogram. |
Intermediate Precision Terazosin RC-A Doxazosin RC-G Doxazosin RC-A Doxazosin RC-B Doxazosin RC-C Doxazosin RC-D Doxazosin Doxazosin RC-E Doxazosin RC-F | Repeatability solutions: 6 spiked Sample solutions at the LOQ level prepared and evaluated by a different analyst on a different day, using a different instrument, and different column serial number | The average recovery at LOQ was within 100 ± 20.0% RSD of the 6 results at LOQ was ≤ 10.0% RSD of the 12 results (for the 2 analysts) at LOQ was ≤ 15.0% |
| Sample Analysis | Sample solution | See Figure 12. |
Solution Stability Terazosin RC-A Doxazosin RC-G Doxazosin RC-A Doxazosin RC-B Doxazosin RC-C Doxazosin RC-D Doxazosin Doxazosin RC-E Doxazosin RC-F | Sensitivity solution and spiked Sample solution at LOQ level. Freshly prepared samples analyzed (t=0). The samples were then stored at 10°C and analyzed periodically for up to 48 h. | Observed changes in the peak area from both solutions for each specified impurity and doxazosin were within ± 10% of the initial time point values. Sensitivity solution was found to be stable at 10° C for a period of 48 h and spiked Sample solution at LOQ level was found to be stable at 10° C for a period of 28 h. |
| Robustness | Robustness solution (0.06 mg/mL of Doxazosin and 0.001 mg/mL for all specified impurities) Changes evaluated: • Flow rate: 0.8 ± 0.1 mL/min • Column temperature: 35° ± 3°C • Variation in isocratic hold time ± 0.5 min. • Variation in the minor component at post gradient: ±2% (absolute value) | System suitability criteria for resolution, tailing factor, and relative retention time met across all evaluated conditions. |
| Impurity Name | RRF |
|---|---|
| Terazosin RC-A | 0.98 |
| Doxazosin RC-G | 1.18 |
| Doxazosin RC-A | 0.97 |
| Doxazosin RC-B | 1.34 |
| Doxazosin RC-C | 1.30 |
| Doxazosin RC-D | 0.64 |
| Doxazosin Mesylate | 1.00 |
| Doxazosin RC-E | 0.94 |
| Doxazosin RC-F | 1.25 |
| RRF values of the impurities were calculated by dividing the slope of the linearity curve for each impurity by the slope of the linearity curve for main component. | |
Forced Degradation Study
According to the available information, Terazosin Related Compound A and Doxazosin Related Compound D are identified as degradation products. The impurities specified in USP monograph procedures, such as Doxazosin Mesylate, and Doxazosin Mesylate tablets, were taken into consideration to establish specificity. The chromatographic method successfully separated all known and unknown impurity peaks from each other, and no coelution was observed with the main peak, ensuring adequate separation of the impurities.
Comment Not Found