Document Type
Emerging StandardPublished date
11/01/2023Input Close Date
To be determinedScientific Experts
Dom Vicchio (Sr. Director, USP)
Introduction
To jump-start the consideration of the potential standards development process and have earlier stakeholder engagement, USP is piloting a new approach for developing and sharing information with our stakeholders. Through a collaboration between USP’s Small Molecules Department and the Global Analytical Development Laboratory, methods will be developed and validated for drug substances and drug products for which no monographs are currently available. The Emerging standards are intended to improve USP’s official standards elaboration process by increasing transparency and allowing for broader stakeholder participation by publishing on the USP website prior to formal notice and comment through publication in the Pharmacopeial Forum.
Doxepin Hydrochloride(HCl) Cream has been evaluated and shown to be a suitable candidate for development under this new program. The methods, in this document, are being published to help analyze Doxepin HCl Cream as a part of USP’s mission to improve global health through public standards that help ensure the safety, quality, and benefit of medicines and foods.
Certain commercial equipment, instruments or materials may be identified in this document to specify adequately the experimental procedure. Such identification does not imply approval, endorsement, or certification by USP of a particular brand or product, nor does it imply that the equipment, instrument, or material is necessarily the best available for the purpose or that any other brand or product was judged to be unsatisfactory or inadequate.
This document is not a USP compendial standard and is intended to serve as a resource for information purposes only. It does not reflect USP or USP’s Expert Body opinions of future revisions to the official text of the USP-NF. Parties relying on the information in this document bear independent responsibility for awareness of, and compliance with, any applicable federal, state, or local laws and requirements.
Background
Doxepin HCl is a benzoxepin derivative and tricyclic antidepressant with antipruritic and sedative activities. Doxepin HCl cream is used to relieve itching from certain skin conditions (such as atopic dermatitis, eczema, neurodermatitis)1. Doxepin is in a class of medications called topical antipruritics. It may work by blocking histamine, a substance in the body that causes certain symptoms, such as itching2.
The USP-NF has a monograph for Doxepin HCl but does not have one for Doxepin HCl cream. Therefore, it was decided to develop methods for Doxepin HCl cream.
This document describes methods and includes supporting chromatographic systems data for peak retention time match and photodiode array (PDA) spectral match, which are suitable for identifying doxepin in the presence of various impurities and excipients. High-performance liquid chromatography (HPLC) methods and supporting validation data, which are suitable for determining strength and purity, are also described.
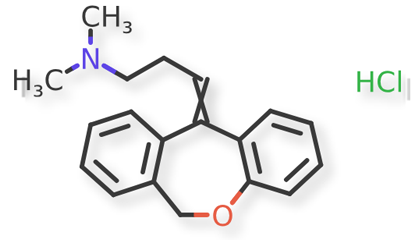
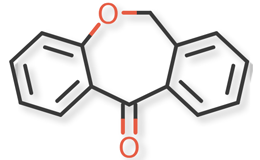
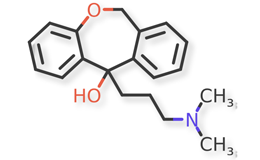
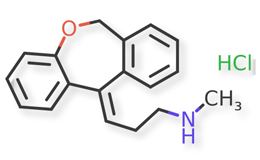
Doxepin HCl and related compounds are shown in Figures 1 and 2.
Figure 2. Doxepin Related Compounds
EXPERIMENTAL
Materials:
USP reference standards of Doxepin HCl and Doxepin related compounds were used.
A Doxepin HCl cream sample with 5% label claim purchased from one commercial source was used to evaluate identification by ultraviolet (UV) spectral match and retention time match, assay, and impurities using methods described in this document.
IDENTIFICATION
Identification of doxepin in Doxepin HCl Cream was evaluated using HPLC-UV with PDA detector and HPLC retention time match.
A. HPLC-UV Spectral Match:
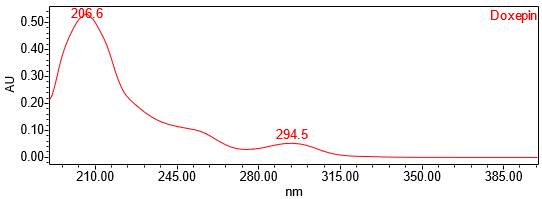
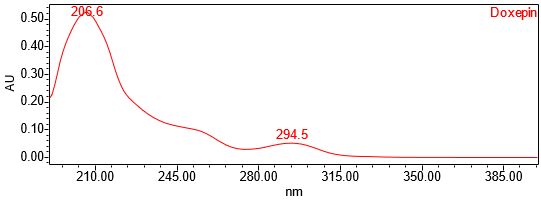
The HPLC assay procedure with PDA detector was used as the chromatographic identification procedure. See the Assay section for the method conditions and solution preparations. The validation parameter and results are summarized in Table 1 and representative UV spectra of the doxepin HCl standard, and sample are shown in Figures 3 and 4.
| Parameter | Samples and Procedure | Results |
|---|---|---|
| Spectral Agreement | Collect PDA from 190-400nm for the Standard solution and Sample solution | The UV spectrum of the doxepin peak from the Sample solution matched the spectrum of doxepin in the Standard solution and exhibited maxima and minima only at the same wavelengths as the Standard solution |
B. Retention Time Match:
The chromatographic retention time is used as an identification method. The HPLC assay procedure was utilized for this identification test. Refer to the Assay section for the method conditions and solution preparations.
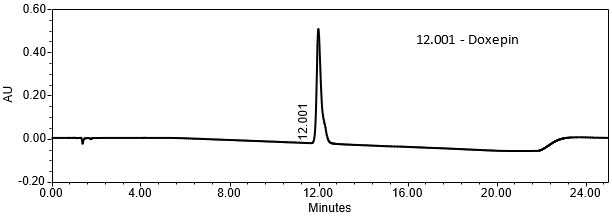
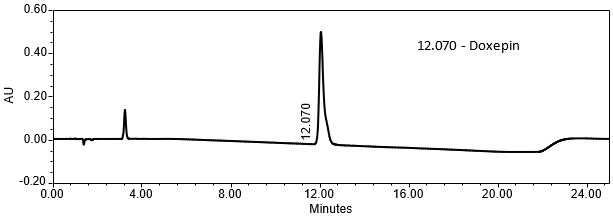
The validation parameter and results are summarized in Table 2, and the example chromatograms for the Standard solution and Sample solution are shown in Figures 5 and 6, respectively.
| Parameter | Samples | Results |
|---|---|---|
| Retention Time Match | Standard solution and Sample solution | The relative standard deviation (RSD) of the doxepin peak retention time for all injections of the Standard solution and Sample solution was <1.0%. |
ASSAY
A gradient reversed phase HPLC method was developed for the assay of Doxepin HCl Cream. The procedure was validated using the criteria described in USP General Chapter <1225>, Validation of Compendial Procedures3 and found to be specific, linear, accurate, precise, rugged, and free from interference for the sample evaluated.
Chemicals:
Ammonium Bicarbonate (BioUltra, ≥99.5% (T)) was obtained from Sigma Aldrich. Optima LC/MS grade acetonitrile and formic acid were obtained from Fisher Scientific. Ultrapure water was obtained from a MilliQ water purification system.
Instruments and method:
The analysis of Doxepin HCl Cream was performed using Waters Alliance 2695 with PDA detector and Agilent 1260 instruments, the results were processed by Empower (Waters software). The Waters Xterra RP18, 4.6mm x 150-mm, 5 µm column was used for analysis. The analysis was performed at 28°C, with a flow rate of 1.0 mL/min and 10 µL as the injection volume. Autosampler temperature was not controlled. The PDA detector was set at 190-400 nm wavelength and the detection of chromatogram was at 207 nm. Separation was achieved by a gradient program as listed in Table 3.
| Time (min) | % Solution A | % Solution B |
|---|---|---|
| 0 | 70 | 30 |
| 3 | 70 | 30 |
| 18 | 50 | 50 |
| 20 | 50 | 50 |
| 20.1 | 70 | 30 |
| 25 | 70 | 30 |
Solutions:
Dilute formic acid: Mix 1 mL of formic acid and 9 mL of water.
Solution A: Weigh 1.6 g of ammonium bicarbonate and dissolve in 1000 mL of water. Adjust pH to 7.0 with Dilute formic acid. Filter the solution through a 0.2-µm membrane filter.
Solution B: Acetonitrile.
Diluent: For 1-L preparation, mix 500 mL of water and 500 mL of acetonitrile.
System Suitability Stock Solution: Weigh and transfer 2 mg each of USP Doxepin Related Compound B RS and USP Doxepin Related Compound C RS into a 10-mL volumetric flask (VF), add 8 mL of Diluent, sonicate for two minutes to dissolve, dilute to volume with Diluent and mix well.
System Suitability Solution: Weigh and transfer 5 mg of USP Doxepin HCl RS into a 10-mL VF, pipette 250 µL of System Suitability Stock solution and dilute to volume with Diluent.
Standard solution: Accurately weigh 10 mg of USP Doxepin HCl RS to 100-mL VF and dilute to volume with Diluent.
Sample solution: Nominally 0.1 mg/mL of doxepin HCl from Doxepin HCl Cream was prepared as follows. Accurately weigh and transfer a portion of Doxepin HCl Cream, equivalent to 2 mg of Doxepin HCl, to a 20mL VF, add about 16 mL of Diluent and vortex for a minute to mix well. Sonicate for 10 minutes and dilute to volume with Diluent. Mix well. Centrifuge the solution at 10000 rpm for 10 minutes and use the supernatant for analysis.
Analytical parameters and validation:
The optimized chromatographic conditions were checked for forced degradation and robustness and validated by evaluating specificity, linearity, accuracy, repeatability, and intermediate precision as described in <1225>3. The system suitability requirements are summarized in Table 4. The validation parameters, solutions, and results for Doxepin HCl Cream are summarized in Table 5. The example chromatograms for the assay Standard solution and Sample solution are shown in Figures 5 and 6, respectively.
| Parameter | Solutions | Results |
|---|---|---|
| Resolution between Doxepin Related Compound B and Doxepin Related Compound C | System Suitability solution | ≥ 2.0. |
| Retention Time Doxepin | Standard solution | About 12 min |
| System Precision (RSD of 5 replicate injections) | Standard solution | ≤ 0.5%. |
| Parameter | Solutions | Results |
|---|---|---|
Specificity (Chromatographic Separation) Peak Purity Analysis (Spectral Homogeneity) | Diluent, Standard solution, and Sample solution | Any peak from the Standard solution and Sample solution was separated from the doxepin peak by a resolution ≥2.0. The PDA data from 190–400 nm showed homogeneity of UV spectrum for the doxepin peak, indicating the lack of coelution. |
| Linearity | Linearity solutions from 50% to 150% of the nominal concentration (0.05, 0.075, 0.10, 0.125, and 0.150 mg/mL of doxepin HCl) | The correlation coefficient was ≥ 0.999. The bias of the linearity curve due to the intercept not being zero was less than ±2.0%. |
| Accuracy | Accuracy solutions from 110–130% of the nominal concentration, triplicate preparations at each level: 110% (0.11 mg/mL), n=3 120% (0.12 mg/mL), n=3 130% (0.13 mg/mL), n=3 | The average recovery at each level was within 100 ± 3.0%. |
| Repeatability | Repeatability solutions: 6 Sample solutions | The RSD of assay results was ≤ 2.0% (n=6). |
| Intermediate Precision | 6 Sample solutions prepared and analyzed by a different analyst, on a different day by using a different instrument and different lot number column | The RSD of assay results was ≤2.0% (n=6). The RSD of assay results was ≤ 3.0% for the combined data of the first and second analysts (n=12). |
| Solution Stability | Standard solution and Sample solution | Standard solution and Sample solution were stable for 24 hours at ambient sample cooler temperature. |
| Sample Assay Test | Sample solution | 101.5% for the one source of the drug product tested. |
Organic Impurities
The HPLC method used for the analysis of the organic impurities is the same as described in the Assay section. The method can be used to quantitate degradants in Doxepin HCl Cream. The procedure was validated using the criteria described in <1225>3 and found to be specific, linear, accurate, precise, rugged, and free from interference for the samples evaluated. The validation study showed that the method was suitable for evaluation of the organic impurities in Doxepin HCl cream.
Solutions:
Prepare Dilute formic acid, Solution A, Solution B, Diluent and System suitability solution and follow chromatographic conditions per the assay procedure.
20% solution: Accurately weigh and transfer 5 mg each of USP Doxepin HCl RS, USP Doxepin Related Compound A RS, USP Doxepin Related Compound B RS and USP Doxepin Related Compound C RS into a 50mL VF, add 40 mL of Diluent, sonicate for 2 minutes to dissolve, dilute to volume with Diluent and mix well.
2% solution: Pipette 2.5 mL of 20% solution to 25-mL VF and dilute to volume with Diluent.
Sensitivity solution: Pipette 500 µL of 2% solution to 10-mL VF and dilute to volume with Diluent.
Sample solution: Nominally 0.5 mg/mL of doxepin HCl from Doxepin HCl Cream was prepared as follows. Accurately weigh and transfer a portion of Doxepin HCl Cream, equivalent to 5 mg of Doxepin HCl, to a 10mL VF, add 8 mL of Diluent and vortex for a minute to mix well. Sonicate for 10 minutes and dilute to volume with Diluent. Mix well. Centrifuge the solution at 10000 rpm for 10 minutes and use the supernatant for analysis.
Analytical parameters and validation:
The optimized chromatographic conditions were validated by evaluating specificity, linearity, accuracy, repeatability, and intermediate precision as described in USP General Chapter <1225>3. Linearity was established for doxepin and related compounds, whereas accuracy and precision were established for doxepin related compounds.
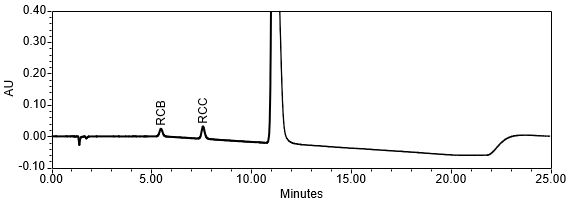
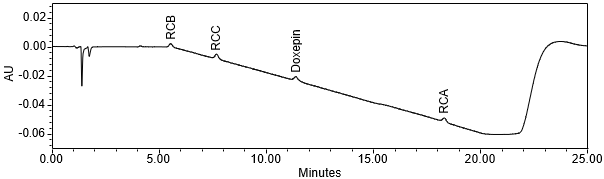
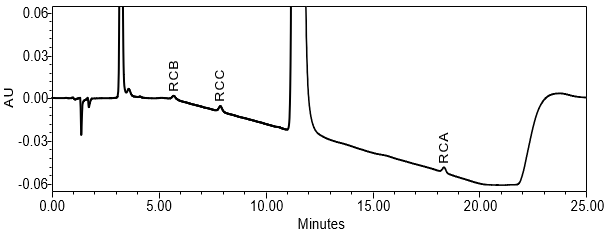
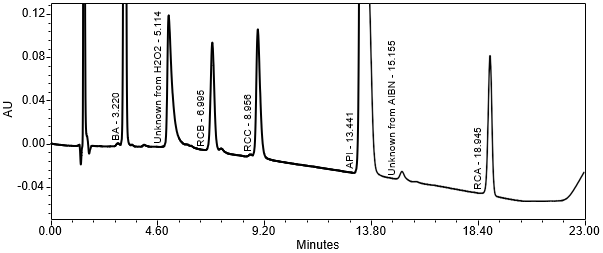
The limit of quantitation (LOQ) was established at 0.10% of sample solution concentration. The system suitability requirements are summarized in Table 6. The validation parameters and results are summarized in Table 7. The example chromatograms of Diluent, System suitability solution, Sensitivity solution, Sample solution with spiked impurities (LOQ) and Sample solution are presented in Figures 7–11, respectively.
| Parameter | Solution | Results |
|---|---|---|
Resolution Resolution between Doxepin Related Compound B and Doxepin Related Compound C | System suitability solution | 6.9 (See Figure 8) |
| Retention Time of Doxepin | Sensitivity solution | 11.5 min |
Relative Retention Time Doxepin Related Compound B Doxepin Related Compound C Doxepin Related Compound A | Sensitivity solution |
0.49 0.68 0.60 (See Figure 9) |
System Precision (RSD of 6 replicate injections) Doxepin Related Compound B Doxepin Related Compound C Doxepin Doxepin Related Compound A | Sensitivity solution |
2.4% 2.1% 3.0% 1.5% |
USP Signal-to-Noise Ratio Doxepin Related Compound B Doxepin Related Compound C Doxepin Doxepin Related Compound A | Sensitivity solution | 52 43 39 50 |
| Parameter | Solutions | Results |
|---|---|---|
| Specificity | Blank (Diluent), Sensitivity solution, and Sample solution | No interference with peaks of interest. Any peak ≥0.1% total area was separated from the main peak by a resolution of ≥2.0, and from adjacent specified impurity peaks by a resolution of ≥1.5. |
LOQ (0.10%) Doxepin Related Compound B Doxepin Related Compound C Doxepin Doxepin Related Compound A | Sensitivity solution and accuracy solution spiked with impurities at the LOQ level. Experimentally determined by selecting the lowest concentration to meet validation signal-to-noise, accuracy, and repeatability criteria. | All concentration values met a signal-to-noise criterion of ≥ 10 (refer to Table 6). See below for accuracy and repeatability. |
Linearity Doxepin Related Compound B Doxepin Related Compound C Doxepin Doxepin Related Compound A | Linearity solutions from LOQ (0.10%) to 1.0% of the nominal concentration of doxepin HCl in Diluent | The correlation coefficient of the linear curves for doxepin and the impurities were not less than 0.99. |
| Relative Response Factor (RRF) Values | Linearity solutions | For results, refer to Table 8. |
Accuracy Doxepin Related Compound B Doxepin Related Compound C Doxepin Related Compound A | Accuracy solutions: impurities spiked in Sample solution at 3 levels: LOQ (0.10%): n=6 0.50% : n=3 1.0% : n=3 | The average recovery for each specified impurity at each level was observed to be within: 0.10% : 100 ± 20.0% 0.50% : 100 ±10.0% 1.0% : 100 ± 5.0% |
Repeatability Doxepin Related Compound B Doxepin Related Compound C Doxepin Related Compound A | Repeatability solutions: 6 spiked Sample solutions at the LOQ level | The RSD of the recovery was ≤ 10.0% (n=6). See Figure 10 for example chromatogram. |
Intermediate Precision Doxepin Related Compound B Doxepin Related Compound C Doxepin Related Compound A | 6 spiked Sample solutions at the LOQ level prepared and analyzed by a different analyst on a different day, using a different instrument and different lot number column | The average recovery at LOQ was within 100 ± 20.0% (for second analyst) RSD of the 6 results at LOQ was ≤ 10.0% (for second analyst). RSD of the 12 results at LOQ from both analysts was ≤ 15.0%. |
| Sample Analysis | Sample solution | See Figure 11. |
Solution Stability Doxepin Related Compound B Doxepin Related Compound C Doxepin Doxepin Relateted Compound A | Sensitivity solution and spiked Sample solution at LOQ level, freshly prepared and analyzed periodically for over 24 hours. | Observed changes in the peak area from both solutions for each specified impurity and doxepin were within ± 10% of the initial time point peak area |
| Impurity Name | RRF |
|---|---|
| Doxepin Related compound A | 0.76 |
| Doxepin Related compound B | 0.59 |
| Doxepin Related compound C | 1.01 |
| Doxepin | 1.00 |
| RRF values of the impurities were calculated by dividing the slope of the linearity curve for each impurity by the slope of the linearity curve for doxepin HCl. | |
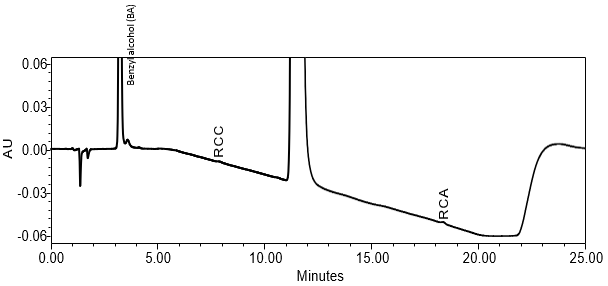
Forced Degradation Study
A forced degradation study was performed by exposing USP Doxepin HCl RS to acid, base, oxidation [ hydrogen peroxide(H2O2) and 2, 2’-Azobis(2-methylpropionitrile)(AIBN)], heat, heat/humidity, and light. The analysis results are included in Table 9.
| Sample | Condition | Doxepin % w/w | Doxepin %TDA | RCA %TDA | RCC %TDA | Unknown %TDA |
|---|---|---|---|---|---|---|
| Control | Freshly prepared | |||||
| Aged Control | Aged for 3 days | 101.1 | 100.0 | ND | ND | |
| Acid Stress | 0.1 N HCI for 3 days | 99.2 | 100.0 | ND | ND | |
| Base Stress | 0.1 N NaOH for 3 days | 101.4 | 100.0 | ND | ND | |
| H2O2 Oxidative Stress_4 h | 3% H2O2 for 4 hours | 88.9 | 94.0 | ND | 0.1 | 5.9 |
| AIBN Oxidative Stress | 0.5 mg/mL AIBN for 3 days at 40° | 82.0 | 94.0 | 0.2 | 0.2 | 5.6 |
| Heat Stress | 105° for 3 days | 101.5 | 100.0 | ND | ND | |
| Heat/Humidity Stress | 80° and 80% RH for 3 days | 97.0 | 99.4 | 0.1 | ND | |
| Photolytic Stress | 600-watt hours/m2 of UV light and 1.2 million lux hours of Visible light | 101.2 | 100.0 | ND | ND | |
| ND – Not Detected | ||||||
No major degradation was detected under acid, base, heat, heat/humidity, and photolytic stress conditions. The H2O2 (3%) stress was first performed for 2 days and about 85% degradation was observed. The H2O2 (3%) stress was later repeated for 4 hours and about 11% degradation was observed, resulting one major degradant. The AIBN stress produced 18% degradation and one major degradant. Both degradant peaks were well separated from the API, three specified impurities, and the excipient benzyl alcohol. Minor RCA and RCC peaks were also observed. The PDA of the API data from 190–400 nm showed homogeneity of the UV spectrum for the doxepin peak, indicating the lack of coelution.
Doxepin peak purity was also investigated using LC/MS adaptable method. The molecular weight of doxepin is 279.4. The protonated molecule (m/z 280.2, [M+H]+) of doxepin was found to be the most abundant ion detected in the control and stress samples. No additional ions were detected at the leading, apex, and trailing portions of the doxepin peak in the stress samples, indicating no co-elution with the doxepin peak. Both the degradant peaks in the H2O2 stress sample and AIBN Stressed Sample had the m/z of 296.2, indicating evidence of oxidation.
Robustness Study
Robustness study was conducted with a Robustness solution (Mixture of doxepin HCl, AIBN Stressed solution, H2O2 Stressed solution, benzyl alcohol, specified impurities). The Robustness solution was analyzed under proposed condition and deliberately changed conditions including flow rate ±10% (1.0 ± 0.1 mL/min), column temperature ±3° (25° ± 3°C), isocratic hold time ±0.5 min (2.0 min ± 0.5 min), post gradient ±2% absolute of Solution B (50% ±2%), and pH of Solution A (7.0 ± 0.2). The system suitability target criteria of relative retention time and USP resolution were met across all evaluated robustness conditions.
This manuscript is dedicated to Mark Tiedje-scientist and valued colleague whose enthusiasm for life and science we’ll always remember.
Comment Not Found