Document Type
Emerging StandardPublished date
11/01/2023Input Close Date
To be determinedScientific Experts
Dom Vicchio (Sr. Director, USP)
Introduction
To jump-start the consideration of the potential standards development process and have earlier stakeholder engagement, USP is piloting a new approach for developing and sharing information with our stakeholders. Through a collaboration between USP’s Small Molecules Department and the Global Analytical Development Laboratory, methods will be developed and validated for drug substances and drug products for which no monographs are currently available. The Emerging standards are intended to improve USP’s official standards elaboration process by increasing transparency and allowing for broader stakeholder participation by publishing on the USP website prior to formal notice and comment through publication in the Pharmacopeial Forum.
Lisdexamfetamine Dimesylate Chewable Tablets has been evaluated and shown to be a suitable candidate for development under this new program. The methods, in this document, are being published to help analyze Lisdexamfetamine Dimesylate Chewable Tablets as a part of USP’s mission to improve global health through public standards that help ensure the safety, quality, and benefit of medicines and foods.
Certain commercial equipment, instruments or materials may be identified in this document to specify adequately the experimental procedure. Such identification does not imply approval, endorsement, or certification by USP of a particular brand or product, nor does it imply that the equipment, instrument, or material is necessarily the best available for the purpose or that any other brand or product was judged to be unsatisfactory or inadequate.
This document is not a USP compendial standard and is intended to serve as a resource for informational purposes only. It does not reflect USP or USP’s Expert Body opinions of future revisions to the official text of the USP-NF. Parties relying on the information in this document bear independent responsibility for awareness of, and compliance with, any applicable federal, state, or local laws and requirements.
Background
Lisdexamfetamine dimesylate is in a class of medications called central nervous system stimulants. Lisdexamfetamine dimesylate is the active ingredient in Lisdexamfetamine Dimesylate Chewable Tablets. It is used as part of a treatment program to control symptoms of attention deficit hyperactivity disorder. Lisdexamfetamine dimesylate is also used to treat an eating disorder characterized by periods of uncontrolled overeating.1
The USP-NF does not have a drug substance monograph for Lisdexamfetamine dimesylate or the drug product monograph for Lisdexamfetamine Dimesylate Chewable Tablets. Therefore, it was decided to develop methods for the drug substance and drug product.
This document describes methods and includes supporting chromatographic systems data for peak retention time match, which are suitable for identifying lisdexamfetamine in the presence of various impurities and excipients. High-performance liquid chromatography (HPLC) methods and supporting validation data, which are suitable for determining strength and purity, are also described.
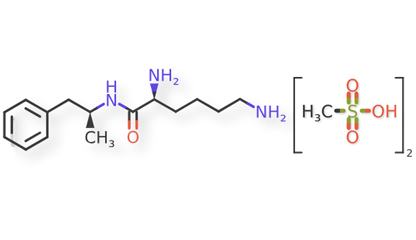
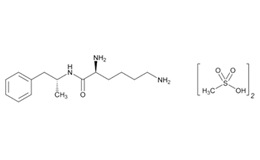
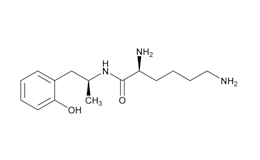
Lisdexamfetamine dimesylate and related impurities are shown in Figures 1 and 2.
EXPERIMENTAL
Materials:
Lisdexamfetamine Dimesylate and impurities were procured from different suppliers.
Lisdexamfetamine Dimesylate Chewable Tablets (10 mg/tab) from a single commercial source was used to evaluate identification by ultraviolet (UV) spectral match and retention time match, assay, and impurities using methods described in this document.
IDENTIFICATION
Identification (ID) of lisdexamfetamine in Lisdexamfetamine Dimesylate Chewable Tablets was evaluated using HPLC-UV spectral match and HPLC retention time match. However, since the UV spectrum of lisdexamfetamine does not exhibit any maxima at the wavelengths above 200 nm, the spectral match may not be suitable.
A. HPLC-UV Spectral Match:
The HPLC assay procedure with photodiode array (PDA) detector was used as the chromatographic identification procedure. See the Assay section for the method conditions and solution preparations. The validation parameter and results are summarized in Table 1 and UV spectra of the Lisdexamfetamine dimesylate standard, and sample are shown in Figures 3 and 4.
| Parameter | Samples and Procedure | Results |
|---|---|---|
| Spectral Agreement | Collect PDA data from 190-400nm for the Standard solution and Sample solution | The UV spectrum of the lisdexamfetamine peak from the Sample solution matched the spectrum of lisdexamfetamine in the Standard solution. No maxima and minima (>200 nm) exhibited; ID by UV spectral match may not be suitable. |
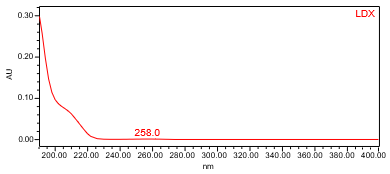
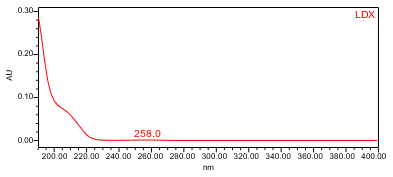
B. Retention Time Match:
The HPLC retention time is used as an identification method. The HPLC assay procedure was utilized for this identification test. Refer to the Assay section for the method conditions and solution preparations.
The validation parameter and results are summarized in Table 2, and the example chromatograms for the Standard solution and Sample solution are shown in Figures 5 and 6, respectively.
| Parameter | Samples | Results |
|---|---|---|
| Retention Time Match | Standard solution and Sample solution | The relative standard deviation (RSD) of the lisdexamfetamine peak retention time for all injections of the Standard solution and Sample solution was not more than (NMT)1.0%. |
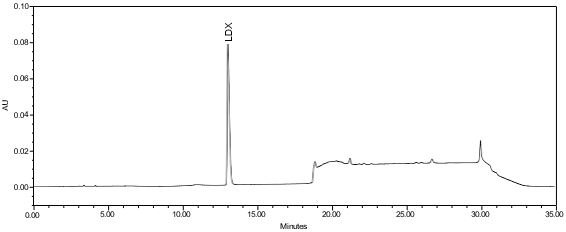
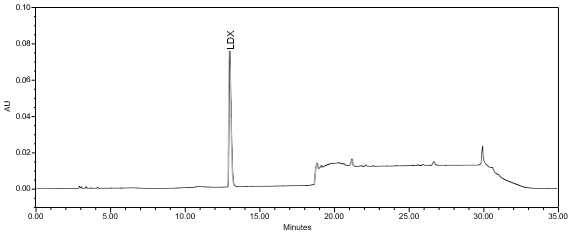
ASSAY
A gradient reversed-phase HPLC procedure was developed for the assay of Lisdexamfetamine Dimesylate Chewable Tablets. The procedure was validated using the criteria described in USP General Chapter <1225>, Validation of Compendial Procedures2 and found to be specific, linear, accurate, precise, robust, and free from interference for the sample evaluated.
Chemicals:
Phosphoric acid, 85% (HPLC grade) and acetonitrile (Optima LC/MS grade) were obtained from Fisher Chemicals. Ultrapure water was obtained from a Milli-Q water purification system.
Instruments and method:
The analysis of Lisdexamfetamine Dimesylate Chewable Tablets was performed using Waters Alliance 2695 and Agilent 1260 instruments with PDA detector, and the results were processed using Empower (Waters software). A YMC Pack ODS-AQ 4.6-mm x 25.0-cm, 5 µm column was used for analysis. The analysis was performed at 42°C, with a flow rate of 0.9 mL/min and 5 µL as the injection volume. Autosampler temperature was kept at ambient. The PDA detector was set at 190-400 nm wavelength and the detection of chromatogram was at 205 nm. Separation was achieved by a gradient program as listed in Table 3.
| Time (min) | Solution A (%) | Solution B (%) |
|---|---|---|
| 0 | 98 | 2 |
| 1 | 98 | 2 |
| 14 | 88.5 | 11.5 |
| 16 | 10 | 90 |
| 26 | 10 | 90 |
| 28 | 98 | 2 |
| 35 | 98 | 2 |
Solutions:
Solution A: 0.1% phosphoric acid in water
Solution B: 0.1% phosphoric acid in acetonitrile
Diluent: Solution A and Solution B (8:2, v/v)
Preparation of System suitability solution: System suitability solution containing 1 mg/mL of lisdexamfetamine dimesylate and 10 µg/mL of Impurity B in Diluent.
Preparation of Standard solution:
Standard solution containing 0.1 mg/mL of lisdexamfetamine dimesylate in Diluent.
Preparation of Sample solution:
Nominally 0.1 mg/mL of lisdexamfetamine dimesylate from Lisdexamfetamine Dimesylate Chewable Tablets was prepared as follows. Accurately weigh and transfer an amount of Lisdexamfetamine Dimesylate Chewable Tablets composite (finely ground NLT 20 tablets), equivalent to 2 mg of lisdexamfetamine dimesylate, into a 20-mL volumetric flask. Add about 18 mL of Diluent and sonicate for 30 minutes with occasional swirling. Dilute to volume with Diluent and mix well. Centrifuge an aliquot of sample solution at 10,000 rpm for 15 minutes. Collect clear supernatant for HPLC injection.
Analytical parameters and validation:
The optimized chromatographic conditions were checked for forced degradation and robustness and validated by evaluating specificity, linearity, precision, and accuracy as described in USP General Chapter <1225>, Validation of Compendial Procedures2. The system suitability requirements are summarized in Table 4. The validation parameters, solutions, and results for Lisdexamfetamine Dimesylate Chewable Tablets are summarized in Table 5. The example chromatograms for the Assay Standard solution and Sample solution are shown in Figures 5 and 6, respectively.
| Parameter | Solutions | Results |
|---|---|---|
| Retention time | Standard solution | About 13.0 min |
| Tailing factor | Standard solution | NMT 2.5 |
| System Precision (for 5 replicate injections) | Standard solution | NMT 0.5% |
| Resolution between Lisdexamfetamine and Impurity B | System suitability solution | Not less than (NLT) 2.0 |
| Parameter | Solutions | Results |
|---|---|---|
Specificity (Chromatographic Separation) Peak Purity Analysis (Spectral Homogeneity) | Diluent, Standard solution, and Sample solution | Any impurity peak from the Standard solution and Sample solution was separated from the lisdexamfetamine peak by a resolution NLT 2.0. The PDA data from 200–400 nm showed homogeneity of UV spectrum for the lisdexamfetamine peak, indicating the lack of coelution. |
| Identification | Standard solution and Sample solution | The RSD for lisdexamfetamine peak retention time from all injections of Standard solution and Sample solutions is NMT 1.0%. |
| Linearity | Linearity solutions from 50% to 150% of the nominal concentration (0.05, 0.075, 0.1, 0.125, and 0.15 mg/mL of lisdexamfetamine dimesylate) | The correlation coefficient was NLT 0.999. The bias of the linearity curve due to the intercept not being zero was less than ± 2.0%. |
| Accuracy | Accuracy solutions from 110–130% of the nominal concentration were prepared in triplicate: 110% (0.11 mg/mL), n=3 120% (0.12 mg/mL), n=3 130% (0.13 mg/mL), n=3 | The average recovery at each level was within 100 ± 3.0%. |
| Repeatability | Repeatability solutions: 6 Sample solutions | The RSD was NMT 2.0% (n=6). |
| Intermediate Precision | Intermediate precision was done by a different analyst, on a different day by using a different instrument and different column lot number. | The RSD was NMT 2.0% (n=6). The RSD was NMT 3.0% for the combined data of the first and second analysts (n=12). |
| Solution Stability | Standard solution and Sample solution | Standard solution and Sample solution were stable for 16 hours at ambient temperature. |
| Sample Assay Test | Sample solution | Sample: 98.9% (n=6) for the one source of the drug product tested. |
ORGANIC IMPURITIES
The HPLC method used for the analysis of the organic impurities is the same as described in the Assay section, except for the gradient program summarized in Table 6. The method can be used to quantitate impurities and degradants in Lisdexamfetamine Dimesylate Chewable Tablets. The procedure was validated using the criteria described in USP General Chapter <1225>, Validation of Compendial Procedures2, and found to be specific, linear, accurate, precise, robust, and free from interference for the samples evaluated. The validation study showed that the method was suitable for the evaluation of the organic impurities in Lisdexamfetamine Dimesylate Chewable Tablets drug product.
| Time (min) | Solution A (%) | Solution B (%) |
|---|---|---|
| 0 | 98 | 2 |
| 1 | 98 | 2 |
| 23 | 80 | 20 |
| 38 | 10 | 90 |
| 43 | 10 | 90 |
| 44 | 98 | 2 |
| 55 | 98 | 2 |
Solutions:
Prepare Solution A, Solution B, Diluent, and System suitability solution as per the Assay procedure.
Preparation of Sensitivity solution:
Sensitivity solution, containing 1 µg/mL each of lisdexamfetamine dimesylate and Impurity M in Diluent.
Preparation of Sample solution:
Nominally 1.0 mg/mL of lisdexamfetamine dimesylate from Lisdexamfetamine Dimesylate Chewable Tablets was prepared as follows. Accurately weigh and transfer an amount of Lisdexamfetamine Dimesylate Chewable Tablets composite (finely ground NLT 20 tablets), equivalent to 20 mg of lisdexamfetamine dimesylate, into a 20-mL volumetric flask. Add about 18 mL of Diluent and sonicate for 30 minutes with occasional swirling. Dilute to volume with Diluent and mix well. Filter a portion through 0.2-µm syringe filter, discard first 2 mL, and collect about 1 mL into HPLC vial for analysis.
Analytical parameters and validation:
The optimized chromatographic conditions were validated by evaluating specificity, linearity, accuracy, repeatability, intermediate precision as described in USP General Chapter <1225>, Validation of Compendial Procedures2. Linearity was established for lisdexamfetamine dimesylate and Impurity M, whereas accuracy and repeatability were established for Impurity M.
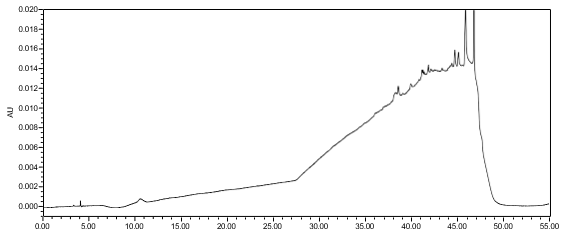
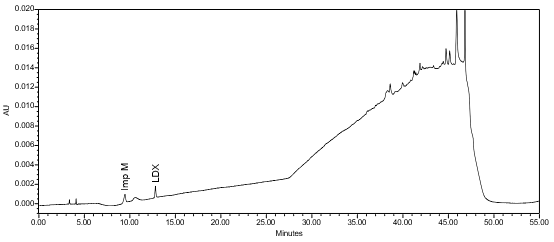
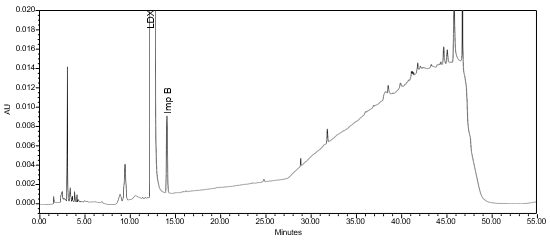
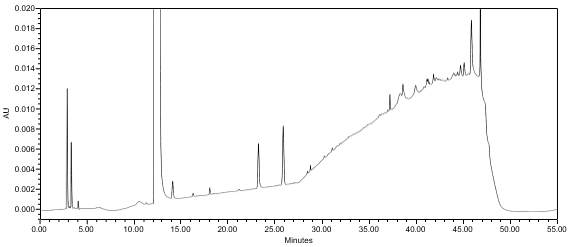
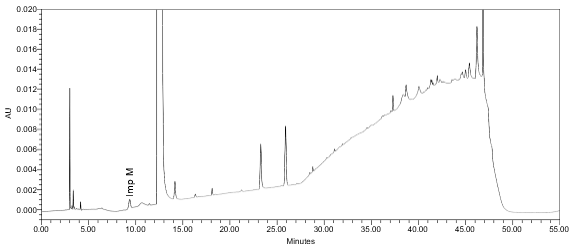
The limit of quantitation (LOQ) was established at 0.10% of sample concentration. The system suitability requirements are summarized in Table 7. The validation parameters and testing results for Lisdexamfetamine Dimesylate Chewable Tablets are summarized in Table 8. The example chromatograms of Diluent, Sensitivity solution, System suitability solution, Sample solution and Sample solution with spiked Impurity M (LOQ), are presented in Figures 7–11, respectively.
| Parameter | Solution | Results |
|---|---|---|
USP Signal-to-Noise Ratio Lisdexamfetamine Impurity M | Sensitivity solution | (See Figure 8) NLT 74 NLT 84 |
Resolution between Lisdexamfetamine and Impurity B | System suitability solution | (See Figure 9) NLT 2.0 |
Relative Retention Time(s) Impurity M Lisdexamfetamine | Sensitivity solution | 0.74 1.00 |
System Precision (RSD of 6 replicate injections) Lisdexamfetamine Impurity M | Sensitivity solution | 1.3% 2.1% |
| Parameter | Solutions | Results |
|---|---|---|
| Specificity | Blank (Diluent), Sample solution, and Spiked solution | No interference between peaks of interest. Any impurity peak ≥0.1% total area was separated from the lisdexamfetamine peak by a resolution of NLT 2.0 and Impurity M peak by a resolution of NLT 1.5. |
LOQ (0.10%) Lisdexamfetamine Dimsesylate Impurity M | Sensitivity solution and accuracy solution spiked with Impurity M at the LOQ level. Experimentally determined using signal-to-noise values, and meeting accuracy, and repeatability criteria at that concentration. | All concentration values met a signal-to-noise criterion of NLT 10 (refer to Table 7). See below for accuracy and repeatability. |
Linearity Lisdexamfetamine Dimesylate Impurity M | Linearity solutions From LOQ (0.10%) to 1.0% of the nominal concentration of lisdexamfetamine dimesylate | The correlation coefficients of the linear curves for lisdexamfetamine dimesylate and Impurity M were NLT 0.99. |
| Relative Response Factor (RRF) Values | Linearity solutions From LOQ (0.10%) to 1.0% of the nominal concentration of lisdexamfetamine dimesylate | For results, refer to Table 9. |
Accuracy Impurity M | Accuracy solutions: Impurity M spiked in Sample solution at 3 levels: LOQ (0.1%): n=6 0.5%: n=3 1.0%: n=3 | The average recovery for Imp M is within: 0.1% level: 100 ± 20.0%. 0.5% level: 100 ±10.0%. 1% level: 100 ± 5.0% |
Repeatability Impurity M | Repeatability solutions: 6 spiked Sample solutions at the LOQ level | RSD of the 6 recoveries is NMT 10.0%. |
Intermediate Precision Impurity M | Repeatability solutions: 6 spiked Sample solutions at the LOQ level prepared and evaluated by a different analyst on a different day, using a different instrument and different column lot number | The average recovery at LOQ was within 100 ± 20.0% (for second analyst) RSD of the 6 results at LOQ was NMT 10.0% (for second analyst). RSD of the 12 results at LOQ from both analysts was NMT 15.0%. |
| Sample Analysis | Sample solution | See Figure 10. |
Solution Stability Lisdexamfetamine Dimesylate Impurity M | LOQ solution and spiked Sample solution at LOQ level. Freshly prepared samples analyzed (t=0) periodically for about 24 hours at ambient temperature. | Peak area changes of lisdexamfetamine dimesylate (in LOQ solution only) and Impurity M peaks (in both solutions) were within ±10% from the initial time point. |
| Impurity Name | RRF |
|---|---|
| Impurity M | 1.43 |
| Lisdexamfetamine Dimesylate | 1.00 |
| RRF value of Impurity M was calculated by dividing the slope of the linearity curve for Impurity M by the slope of the linearity curve for Lisdexamfetamine Dimesylate. | |
Comment Not Found